Health
Brain exposure to SARS-CoV-2 virions perturbs synaptic homeostasis
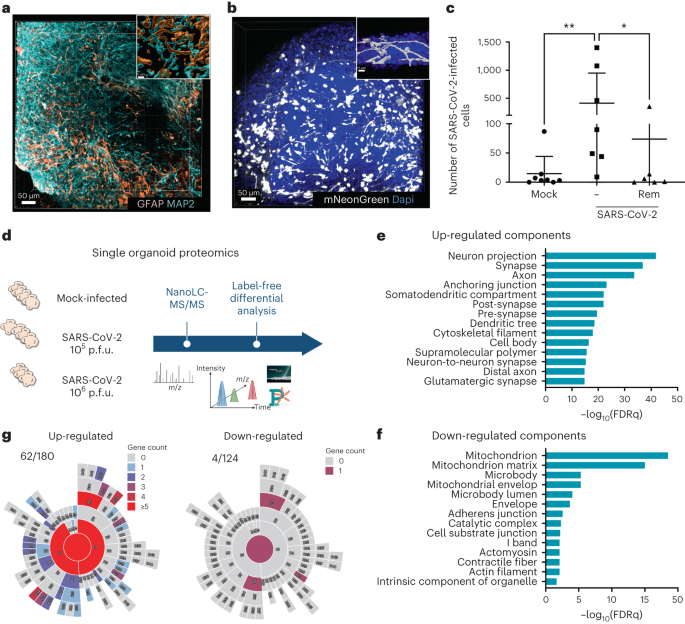
Gavriatopoulou, M. et al. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 20, 493–506 (2020).
Salinas, S. & Simonin, Y. [Neurological damage linked to coronaviruses: SARS-CoV-2 and other human coronaviruses]. Med.Sci. (Paris) 36, 775–782 (2020).
Koralnik, I. J. & Tyler, K. L. COVID-19: a global threat to the nervous system. Ann. Neurol. 88, 1–11 (2020).
Iadecola, C., Anrather, J. & Kamel, H. Effects of COVID-19 on the nervous system. Cell 183, 16–27 e11 (2020).
Helms, J. et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit. Care 24, 491 (2020).
Varatharaj, A. et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 7, 875–882 (2020).
Rogers, J. P. et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 7, 611–627 (2020).
Nagu, P., Parashar, A., Behl, T. & Mehta, V. CNS implications of COVID-19: a comprehensive review. Rev. Neurosci. 32, 219–234 (2021).
Baker, H. A., Safavynia, S. A. & Evered, L. A. The ‘third wave’: impending cognitive and functional decline in COVID-19 survivors. Br. J. Anaesth. 126, 44–47 (2021).
Taquet, M., Geddes, J. R., Husain, M., Luciano, S. & Harrison, P. J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 8, 416–427 (2021).
Hellmuth, J. et al. Persistent COVID-19-associated neurocognitive symptoms in non-hospitalized patients. J. Neurovirol. 27, 191–195 (2021).
Douaud, G. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature https://doi.org/10.1038/s41586-022-04569-5 (2022)
Blazhenets, G. et al. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J. Nucl. Med. 62, 910–915 (2021).
Taquet, M. et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 9, 815–827 (2022).
Monje, M. & Iwasaki, A. The neurobiology of long COVID. Neuron 110, 3484–3496 (2022).
Stein, S. R. et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 612, 758–763 (2022).
Ramani, A., Pranty, A. I. & Gopalakrishnan, J. Neurotropic effects of SARS-CoV-2 modeled by the human brain organoids. Stem Cell Rep. 16, 373–384 (2021).
Song, E. et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 218, e20202135 (2021).
Qian, X., Song, H. & Ming, G. L. Brain organoids: advances, applications and challenges. Development 146, dev166074 (2019).
Hodge, R. D. et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68 (2019).
Brola, W. & Wilski, M. Neurological consequences of COVID-19. Pharmacol. Rep. 74, 1208–1222 (2022).
Antony, A. R. & Haneef, Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure 83, 234–241 (2020).
Kubota, T., Gajera, P. K. & Kuroda, N. Meta-analysis of EEG findings in patients with COVID-19. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2020.107682 (2020).
Lin, L. et al. Electroencephalographic abnormalities are common in COVID-19 and are associated with outcomes. Ann. Neurol. 89, 872–883 (2021).
Yang, A. C. et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 595, 565–571 (2021).
Samudyata et al. SARS-CoV-2 promotes microglial synapse elimination in human brain organoids. Mol. Psychiatry https://doi.org/10.1038/s41380-022-01786-2 (2022).
Partiot, E. et al. Organotypic culture of human brain explants as a preclinical model for AI-driven antiviral studies. EMBO Mol. Med. https://doi.org/10.1038/s44321-024-00039-9 (2024).
O’Sullivan, M. L. et al. FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron 73, 903–910 (2012).
Sando, R. & Sudhof, T. C. Latrophilin GPCR signaling mediates synapse formation. Elife 10, e65717 (2021).
Rothe, J. et al. Involvement of the adhesion GPCRs latrop–hilins in the regulation of insulin release. Cell Rep. 26, 1573–1584 e1575 (2019).
Ramani, A. et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 39, e106230 (2020).
Ferren, M. et al. Hamster organotypic modeling of SARS-CoV-2 lung and brainstem infection. Nat. Commun. 12, 5809 (2021).
Bauer, L. et al. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 45, 358–368 (2022).
Zivaljic, M., et al. Poor sensitivity of iPSC-derived neural progenitors and glutamatergic neurons to SARS-CoV-2. Preprint at bioRxiv https://doi.org/10.1101/2022.07.25.501370 (2022)
Koopmans, F. et al. SynGO: an evidence-based, expert-curated knowledge base for the synapse. Neuron 103, 217–234 e214 (2019).
Beckman, D. et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep. 41, 111573 (2022).
Xie, X. et al. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe 27, 841–848 e843 (2020).
Corman, V. M. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 25, 2000045 (2020).
Fernandez-Rodriguez, A. et al. Post-mortem microbiology in sudden death: sampling protocols proposed in different clinical settings. Clin. Microbiol. Infect. 25, 570–579 (2019).
Burbach, J. P. H. & Meijer, D. H. Latrophilin’s social protein network. Front. Neurosci. 13, 643 (2019).
Sando, R., Jiang, X. & Sudhof, T. C. Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science 363, eaav7969 (2019).
Bielarz, V. et al. Susceptibility of neuroblastoma and glioblastoma cell lines to SARS-CoV-2 infection. Brain Res. 1758, 147344 (2021).
Fontes-Dantas, F. L. et al. SARS-CoV-2 spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. 42, 112189 (2023).
May, D. G. et al. A BioID-derived proximity interactome for SARS-CoV-2 proteins. Viruses https://doi.org/10.3390/v14030611 (2022).
Bakhache, W., et al. Pharmacological perturbation of intracellular dynamics as a SARS-CoV-2 antiviral strategy. Preprint at bioRxiv https://doi.org/10.1101/2021.09.10.459410 (2021)
Prasad, V. & Bartenschlager, R. A snapshot of protein trafficking in SARS-CoV-2 infection. Biol. Cell. https://doi.org/10.1111/boc.202200073 (2022).
Jouvenet, N., Goujon, C. & Banerjee, A. Clash of the titans: interferons and SARS-CoV-2. Trends Immunol. 42, 1069–1072 (2021).
Silva, M. M. et al. MicroRNA-186-5p controls GluA2 surface expression and synaptic scaling in hippocampal neurons. Proc. Natl Acad. Sci. USA 116, 5727–5736 (2019).
Schanzenbacher, C. T., Langer, J. D. & Schuman, E. M. Time- and polarity-dependent proteomic changes associated with homeostatic scaling at central synapses. Elife 7, e33322 (2018).
Dubes, S. et al. miR-124-dependent tagging of synapses by synaptopodin enables input-specific homeostatic plasticity. EMBO J. 41, e109012 (2022).
Sun, Z. et al. Mass spectrometry analysis of newly emerging coronavirus HCoV-19 spike protein and human ACE2 reveals camouflaging glycans and unique post-translational modifications. Engineering 7, 1441–1451 (2021).
Lorenzo, R. et al. Deamidation drives molecular aging of the SARS-CoV-2 spike protein receptor-binding motif. J. Biol. Chem. 297, 101175 (2021).
Zhao, J., Li, J., Xu, S. & Feng, P. Emerging roles of protein deamidation in innate immune signaling. J. Virol. 90, 4262–4268 (2016).
Arcos-Burgos, M. et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol. Psychiatry 15, 1053–1066 (2010).
Lange, M. et al. The ADHD-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol. Psychiatry 17, 946–954 (2012).
Regan, S. L. et al. A novel role for the ADHD risk gene latrophilin-3 in learning and memory in Lphn3 knockout rats. Neurobiol. Dis. 158, 105456 (2021).
Domene, S. et al. Screening of human LPHN3 for variants with a potential impact on ADHD susceptibility. Am. J. Med. Genet. B 156B, 11–18 (2011).
Orsini, C. A. et al. Behavioral and transcriptomic profiling of mice null for Lphn3, a gene implicated in ADHD and addiction. Mol. Genet. Genomic Med. 4, 322–343 (2016).
Wallis, D. et al. Initial characterization of mice null for Lphn3, a gene implicated in ADHD and addiction. Brain Res. 1463, 85–92 (2012).
Li, J. et al. Alternative splicing controls teneurin-latrophilin interaction and synapse specificity by a shape-shifting mechanism. Nat. Commun. 11, 2140 (2020).
Giandomenico, S. L. et al. Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 22, 669–679 (2019).
Trujillo, C. A. et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, 558–569 e557 (2019).
Chaumont, H. et al. Long-term outcomes after NeuroCOVID: a 6-month follow-up study on 60 patients. Rev. Neurol. 178, 137–143 (2022).
Coulter, M. E. et al. The ESCRT-III protein CHMP1A mediates secretion of sonic hedgehog on a distinctive subtype of extracellular vesicles. Cell Rep. 24, 973–986 e978 (2018).
Gee, G. V., Manley, K. & Atwood, W. J. Derivation of a JC virus-resistant human glial cell line: implications for the identification of host cell factors that determine viral tropism. Virology 314, 101–109 (2003).
Rebendenne, A. et al. SARS-CoV-2 triggers an MDA-5-dependent interferon response which is unable to control replication in lung epithelial cells. J. Virol. 95, e02415–e02420 (2021).
Bouyssie, D. et al. Proline: an efficient and user-friendly software suite for large-scale proteomics. Bioinformatics 36, 3148–3155 (2020).
Wieczorek, S., Combes, F., Borges, H. & Burger, T. Protein-level statistical analysis of quantitative label-free proteomics data with ProStaR. Methods Mol. Biol. 1959, 225–246 (2019).
Hulstaert, N. et al. ThermoRawFileParser: modular, scalable, and cross-platform RAW file conversion. J. Proteome Res. 19, 537–542 (2020).
Degroeve, S., et al. ionbot: a novel, innovative and sensitive machine learning approach to LC-MS/MS peptide identification. Preprint at bioRxiv https://doi.org/10.1101/2021.07.02.450686 (2021).
Lutz, W. WillyLutz/electrical-analysis-sars-cov-2. GitHub https://github.com/WillyLutz/electrical-analysis-sars-cov-2 (2024).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
|
Sources 2/ https://www.nature.com/articles/s41564-024-01657-2 The mention sources can contact us to remove/changing this article |
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: [email protected]: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
to request, modification Contact us at Here or [email protected]



