Health
Molecular and cellular mechanisms of itch sensation and the anti-itch drug targets
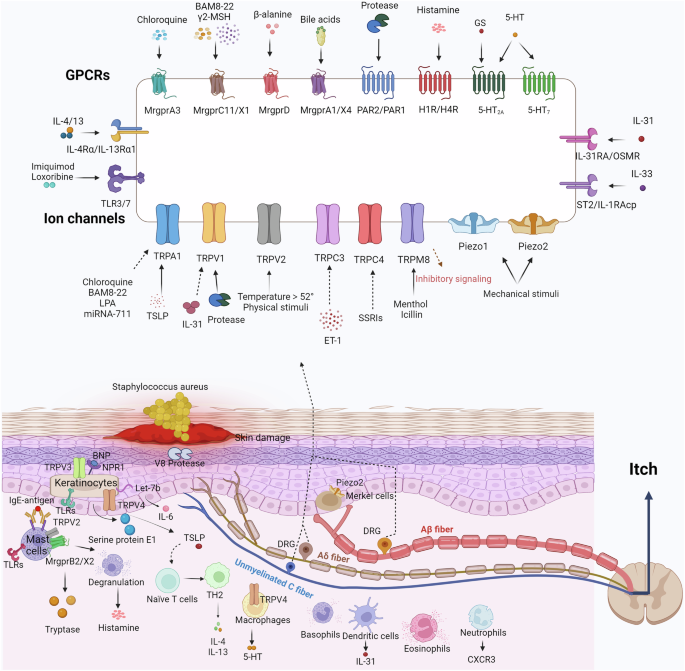
Roh YS, Choi J, Sutaria N, Kwatra SG. Itch: Epidemiology, clinical presentation, and diagnostic workup. J Am Acad Dermatol. 2022;86:1–14.
Steinhoff M, Al-Khawaga S, Buddenkotte J. Itch in elderly patients: Origin, diagnostics, management. J Allergy Clin Immunol. 2023;152:42–49.
Hawro T, Hawro M, Zalewska-Janowska A, Weller K, Metz M, Maurer M. Pruritus and sleep disturbances in patients with psoriasis. Arch Dermatol Res. 2020;312:103–11.
Leader B, Carr CW, Chen SC. Pruritus epidemiology and quality of life. Handb Exp Pharmacol. 2015;226:15–38.
Weisshaar E, Szepietowski JC, Dalgard FJ, Garcovich S, Gieler U, Giménez-Arnau AM, et al. European S2k guideline on chronic pruritus. Acta Derm Venereol. 2019;99:469–506.
Stefaniak AA, Krajewski PK, Bednarska-Chabowska D, Bolanowski M, Mazur G, Szepietowski JC. Itch in adult population with type 2 diabetes mellitus: clinical profile, pathogenesis and disease-related burden in a cross-sectional study. Biology. 2021;10:1332.
Hu X, Sang Y, Yang M, Chen X, Tang W. Prevalence of chronic kidney disease-associated pruritus among adult dialysis patients: A meta-analysis of cross-sectional studies. Medicine. 2018;97:e10633.
Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–32.
Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–65.
Lembo PM, Grazzini E, Groblewski T, O’Donnell D, Roy MO, Zhang J, et al. Proenkephalin A gene products activate a new family of sensory neuron–specific GPCRs. Nat Neurosci. 2002;5:201–9.
Heller D, Doyle JR, Raman VS, Beinborn M, Kumar K, Kopin AS. Novel probes establish Mas-related G protein-coupled receptor X1 variants as receptors with loss or gain of function. J Pharmacol Exp Ther. 2016;356:276–83.
Klein A, Solinski HJ, Malewicz NM, Ieong HF, Sypek EI, Shimada SG, et al. Pruriception and neuronal coding in nociceptor subtypes in human and nonhuman primates. Elife. 2021;10:e64506.
Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal. 2011;4:ra45.
Guan Y, Liu Q, Tang Z, Raja SN, Anderson DJ, Dong X. Mas-related G-protein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci USA. 2010;107:15933–8.
Li Z, He SQ, Xu Q, Yang F, Tiwari V, Liu Q, et al. Activation of MrgC receptor inhibits N-type calcium channels in small-diameter primary sensory neurons in mice. Pain. 2014;155:1613–21.
Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25.
Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, et al. Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem. 2004;279:23559–64.
Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, et al. Mechanisms of itch evoked by β-alanine. J Neurosci. 2012;32:14532–7.
Qu L, Fan N, Ma C, Wang T, Han L, Fu K, et al. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain. 2014;137:1039–50.
Chompunud Na Ayudhya C, Roy S, Thapaliya M, Ali H. Roles of a mast cell-specific receptor MRGPRX2 in host defense and inflammation. J Dent Res. 2020;99:882–90.
Thapaliya M, Chompunud Na Ayudhya C, Amponnawarat A, Roy S, Ali H. Mast cell-specific MRGPRX2: a key modulator of neuro-immune interaction in allergic diseases. Curr Allergy Asthma Rep. 2021;21:3.
Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of mast-cell-expressed Mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity. 2019;50:1163–71.e5.
Jia T, Che D, Zheng Y, Zhang H, Li Y, Zhou T, et al. Mast cells initiate type 2 inflammation through tryptase released by MRGPRX2/MRGPRB2 activation in atopic dermatitis. J Invest Dermatol. 2024;144:53–62.e2.
Yu H, Zhao T, Liu S, Wu Q, Johnson O, Wu Z, et al. MRGPRX4 is a bile acid receptor for human cholestatic itch. Elife. 2019;8:e48431.
Meixiong J, Vasavda C, Green D, Zheng Q, Qi L, Kwatra SG, et al. Identification of a bilirubin receptor that may mediate a component of cholestatic itch. Elife. 2019;8:e44116.
Meixiong J, Vasavda C, Snyder SH, Dong X. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc Natl Acad Sci USA. 2019;116:10525–30.
Zhang L, Taylor N, Xie Y, Ford R, Johnson J, Paulsen JE, et al. Cloning and expression of MRG receptors in macaque, mouse, and human. Brain Res Mol Brain Res. 2005;133:187–97.
Bender E, Buist A, Jurzak M, Langlois X, Baggerman G, Verhasselt P, et al. Characterization of an orphan G protein-coupled receptor localized in the dorsal root ganglia reveals adenine as a signaling molecule. Proc Natl Acad Sci USA. 2002;99:8573–8.
Cox PJ, Pitcher T, Trim SA, Bell CH, Qin W, Kinloch RA. The effect of deletion of the orphan G-protein coupled receptor (GPCR) gene MrgE on pain-like behaviours in mice. Mol Pain. 2008;4:2.
Shen Q, Han Y, Wu K, He Y, Jiang X, Liu P, et al. MrgprF acts as a tumor suppressor in cutaneous melanoma by restraining PI3K/Akt signaling. Signal Transduct Target Ther. 2022;7:147.
Gembardt F, Grajewski S, Vahl M, Schultheiss HP, Walther T. Angiotensin metabolites can stimulate receptors of the Mas-related genes family. Mol Cell Biochem. 2008;319:115–23.
Tsagareli MG, Nozadze I. An overview on transient receptor potential channels superfamily. Behav Pharmacol. 2020;31:413–34.
Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–7.
Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–60.
Wang G, Savinko T, Wolff H, Dieu-Nosjean MC, Kemeny L, Homey B, et al. Repeated epicutaneous exposures to ovalbumin progressively induce atopic dermatitis-like skin lesions in mice. Clin Exp Allergy. 2007;37:151–61.
Zhu Y, Pan WH, Wang XR, Liu Y, Chen M, Xu XG, et al. Tryptase and protease-activated receptor-2 stimulate scratching behavior in a murine model of ovalbumin-induced atopic-like dermatitis. Int Immunopharmacol. 2015;28:507–12.
Lee KP, Koshelev MV. Upcoming topical TRPV1 anti-pruritic compounds. Dermatol Online J. 2020;26:13030/qt188477hq.
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–41.
Bíró T, Tóth BI, Marincsák R, Dobrosi N, Géczy T, Paus R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim Biophys Acta. 2007;1772:1004–21.
Zhang D, Spielmann A, Wang L, Ding G, Huang F, Gu Q, et al. Mast-cell degranulation induced by physical stimuli involves the activation of transient-receptor-potential channel TRPV2. Physiol Res. 2012;61:113–24.
Nattkemper LA, Tey HL, Valdes-Rodriguez R, Lee H, Mollanazar NK, Albornoz C, et al. The genetics of chronic itch: gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. J Invest Dermatol. 2018;138:1311–7.
Yang P, Zhu MX. TRPV3. Handb Exp Pharmacol. 2014;222:273–91.
Mahmoud O, Soares GB, Yosipovitch G. Transient receptor potential channels and itch. Int J Mol Sci. 2022;24:420.
Zhao J, Munanairi A, Liu XY, Zhang J, Hu L, Hu M, et al. PAR2 mediates itch via TRPV3 signaling in keratinocytes. J Invest Dermatol. 2020;140:1524–32.
Shirolkar P, Mishra SK. Role of TRP ion channels in pruritus. Neurosci Lett. 2022;768:136379.
Han Y, Luo A, Kamau PM, Takomthong P, Hu J, Boonyarat C, et al. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br J Pharmacol. 2021;178:1669–83.
Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90:558–64.
Tsagareli MG, Follansbee T, Iodi Carstens M, Carstens E. Targeting transient receptor potential (TRP) channels, Mas-related G-protein-coupled receptors (Mrgprs), and protease-activated receptors (PARs) to relieve itch. Pharmaceuticals. 2023;16:1707.
Larkin C, Chen W, Szabó IL, Shan C, Dajnoki Z, Szegedi A, et al. Novel insights into the TRPV3-mediated itch in atopic dermatitis. J Allergy Clin Immunol. 2021;147:1110–4. e5
Zhang Q, Henry G, Chen Y. Emerging role of transient receptor potential vanilloid 4 (TRPV4) ion channel in acute and chronic itch. Int J Mol Sci. 2021;22:7591.
Luo J, Feng J, Yu G, Yang P, Mack MR, Du J, et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J Allergy Clin Immunol. 2018;141:608–19.e7.
Yan J, Ye F, Ju Y, Wang D, Chen J, Zhang X, et al. Cimifugin relieves pruritus in psoriasis by inhibiting TRPV4. Cell Calcium. 2021;97:102429.
Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27:3549–63.
Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–95.
Chen Y, Wang ZL, Yeo M, Zhang QJ, López-Romero AE, Ding HP, et al. Epithelia-sensory neuron cross talk underlies cholestatic itch induced by lysophosphatidylcholine. Gastroenterology. 2021;161:301–17.e16.
Liu Y, Liu Y, Limjunyawong N, Narang C, Jamaldeen H, Yu S, et al. Sensory neuron-expressed TRPC3 mediates acute and chronic itch. Pain. 2023;164:98–110.
Morita T, McClain SP, Batia LM, Pellegrino M, Wilson SR, Kienzler MA, et al. HTR7 mediates serotonergic acute and chronic itch. Neuron. 2015;87:124–38.
Xie Z, Hu H. TRP channels as drug targets to relieve itch. Pharmceuticals. 2018;11:100.
Lee SH, Cho PS, Tonello R, Lee HK, Jang JH, Park GY, et al. Peripheral serotonin receptor 2B and transient receptor potential channel 4 mediate pruritus to serotonergic antidepressants in mice. J Allergy Clin Immunol. 2018;142:1349–52.e16.
Liu Y, Mikrani R, He Y, Faran Ashraf Baig MM, Abbas M, Naveed M, et al. TRPM8 channels: a review of distribution and clinical role. Eur J Pharmacol. 2020;882:173312.
Palkar R, Ongun S, Catich E, Li N, Borad N, Sarkisian A, et al. Cooling relief of acute and chronic itch requires TRPM8 channels and neurons. J Invest Dermatol. 2018;138:1391–9.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Liu T, Berta T, Xu ZZ, Park CK, L, Zhang N, et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195–207.
Szöllősi AG, McDonald I, Szabó IL, Meng J, van den Bogaard E, Steinhoff M. TLR3 in chronic human itch: a keratinocyte-associated mechanism of peripheral itch sensitization. J Invest Dermatol. 2019;139:2393–6.e6.
Wang ZH, Feng Y, Hu Q, Wang XL, Zhang L, Liu TT, et al. Keratinocyte TLR2 and TLR7 contribute to chronic itch through pruritic cytokines and chemokines in mice. J Cell Physiol. 2023;238:257–73.
Gangwar RS, Gudjonsson JE, Ward NL. Mouse models of psoriasis: a comprehensive review. J Invest Dermatol. 2022;142:884–97.
Min H, Lee H, Lim H, Jang YH, Chung SJ, Lee CJ, et al. TLR4 enhances histamine-mediated pruritus by potentiating TRPV1 activity. Mol Brain. 2014;7:59.
Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, et al. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med. 2015;21:1326–31.
Pan H, Fatima M, Li A, Lee H, Cai W, Horwitz L, et al. Identification of a spinal circuit for mechanical and persistent spontaneous itch. Neuron. 2019;103:1135–49.e6.
Winkler CW, Taylor KG, Peterson KE. Location is everything: let-7b microRNA and TLR7 signaling results in a painful TRP. Sci Signal. 2014;7:pe14.
Wu Y, Liu L, Bian C, Diao Q, Nisar MF, Jiang X, et al. MicroRNA let-7b inhibits keratinocyte differentiation by targeting IL-6 mediated ERK signaling in psoriasis. Cell Commun Signal. 2018;16:58.
Hill RZ, Loud MC, Dubin AE, Peet B, Patapoutian A. PIEZO1 transduces mechanical itch in mice. Nature. 2022;607:104–10.
Feng J, Luo J, Yang P, Du J, Kim BS, Hu H. Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science. 2018;360:530–3.
Auyeung KL, Kim BS. Emerging concepts in neuropathic and neurogenic itch. Ann Allergy Asthma Immunol. 2023;131:561–6.
Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA. 2009;106:11330–5.
He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92–96.
Dressler C, Rosumeck S, Werner RN, Magerl M, Metz M, Maurer M, et al. Executive summary of the methods report for ‘The EAACI/GA(2) LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. The 2017 Revision and Update’. Allergy. 2018;73:1145–6.
Huang J, Polgár E, Solinski HJ, Mishra SK, Tseng PY, Iwagaki N, et al. Circuit dissection of the role of somatostatin in itch and pain. Nat Neurosci. 2018;21:707–16.
Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–53.
Meng QT, Liu XY, Liu XT, Liu J, Munanairi A, Barry DM, et al. BNP facilitates NMB-encoded histaminergic itch via NPRC-NMBR crosstalk. Elife. 2021;10:e71689.
Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–3.
Liu X, Wang Y, Tao T, Zeng L, Wang D, Wen Y, et al. GRPR/extracellular signal-regulated kinase and NPRA/extracellular signal-regulated kinase signaling pathways play a critical role in spinal transmission of chronic itch. J Invest Dermatol. 2021;141:863–73.
Liu X, Wang D, Wen Y, Zeng L, Li Y, Tao T, et al. Spinal GRPR and NPRA contribute to chronic itch in a murine model of allergic contact dermatitis. J Invest Dermatol. 2020;140:1856–66.e7
Mu D, Deng J, Liu KF, Wu ZY, Shi YF, Guo WM, et al. A central neural circuit for itch sensation. Science. 2017;357:695–9.
Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons Co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217–28.e13.
Campion M, Smith L, Gatault S, Métais C, Buddenkotte J, Steinhoff M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp Dermatol. 2019;28:1501–4.
Du LX, Zhu JY, Mi WL. Cytokines and chemokines modulation of itch. Neuroscience. 2022;495:74–85.
Lu J, Wu K, Zeng Q, Xiang Y, Gao L, Huang J. Serum interleukin-31 level and pruritus in atopic dermatitis: A Meta-analysis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43:124–30.
Misery L, Pierre O, Le Gall-Ianotto C, Lebonvallet N, Chernyshov PV, Le Garrec R, et al. Basic mechanisms of itch. J Allergy Clin Immunol. 2023;152:11–23.
Meng J, Moriyama M, Feld M, Buddenkotte J, Buhl T, Szöllösi A, et al. New mechanism underlying IL-31-induced atopic dermatitis. J Allergy Clin Immunol. 2018;141:1677–89.e8.
Okano M, Hirahara K, Kiuchi M, Onoue M, Iwamura C, Kokubo K, et al. Interleukin-33-activated neuropeptide CGRP-producing memory Th2 cells cooperate with somatosensory neurons to induce conjunctival itch. Immunity. 2022;55:2352–68.e7.
Trier AM, Mack MR, Fredman A, Tamari M, Ver Heul AM, Zhao Y, et al. IL-33 signaling in sensory neurons promotes dry skin itch. J Allergy Clin Immunol. 2022;149:1473–80.e6.
Grenier A, Combaux D, Chastre J, Gougerot-Pocidalo MA, Gibert C, Dehoux M, et al. Oncostatin M production by blood and alveolar neutrophils during acute lung injury. Lab Invest. 2001;81:133–41.
Tseng PY, Hoon MA. Oncostatin M can sensitize sensory neurons in inflammatory pruritus. Sci Transl Med. 2021;13:eabe3037.
Lin W, Zhou Q, Liu C, Ying M, Xu S. Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Sci Rep. 2017;7:17797.
Walsh CM, Hill RZ, Schwendinger-Schreck J, Deguine J, Brock EC, Kucirek N, et al. Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. Elife. 2019;8:e48448.
Svanberg S, Li Z, Öhlund P, Roy A, Åbrink M. Mast cells limit ear swelling independently of the chymase mouse mast cell protease 4 in an MC903-induced atopic dermatitis-like mouse model. Int J Mol Sci. 2020;21:6311.
Mishra SK, Wheeler JJ, Pitake S, Ding H, Jiang C, Fukuyama T, et al. Periostin activation of integrin receptors on sensory neurons induces allergic itch. Cell Rep. 2020;31:107472.
Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–66.
Sanjel B, Kim BH, Song MH, Carstens E, Shim WS. Glucosylsphingosine evokes pruritus via activation of 5-HT2A receptor and TRPV4 in sensory neurons. Br J Pharmacol. 2022;179:2193–207.
Deng L, Costa F, Blake KJ, Choi S, Chandrabalan A, Yousuf MS, et al. S. aureus drives itch and scratch-induced skin damage through a V8 protease-PAR1 axis. Cell. 2023;186:5375–93.e25.
Rochman Y, Dienger-Stambaugh K, Richgels PK, Lewkowich IP, Kartashov AV, Barski A, et al. TSLP signaling in CD4+ T cells programs a pathogenic T helper 2 cell state. Sci Signal. 2018;11:eaam8858.
Nakashima C, Ishida Y, Kitoh A, Otsuka A, Kabashima K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp Dermatol. 2019;28:1405–11.
Sun S, Xu Q, Guo C, Guan Y, Liu Q, Dong X. Leaky gate model: intensity-dependent coding of pain and itch in the spinal cord. Neuron. 2017;93:840–53.e5.
Lay M, Dong X. Neural mechanisms of itch. Annu Rev Neurosci. 2020;43:187–205.
Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–98.
Acton D, Ren X, Di Costanzo S, Dalet A, Bourane S, Bertocchi I, et al. Spinal neuropeptide Y1 receptor-expressing neurons form an essential excitatory pathway for mechanical itch. Cell Rep. 2019;28:625–39.e6.
Ren X, Liu S, Virlogeux A, Kang SJ, Brusch J, Liu Y, et al. Identification of an essential spinoparabrachial pathway for mechanical itch. Neuron. 2023;111:1812–29.e6.
Gao ZR, Chen WZ, Liu MZ, Chen XJ, Wan L, Zhang XY, et al. Tac1-expressing neurons in the periaqueductal gray facilitate the itch-scratching cycle via descending regulation. Neuron. 2019;101:45–59.e9.
Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron. 2018;98:482–94.
Deng J, Zhou H, Lin JK, Shen ZX, Chen WZ, Wang LH, et al. The parabrachial nucleus directly channels spinal nociceptive signals to the intralaminar thalamic nuclei, but not the amygdala. Neuron. 2020;107:909–23.e6.
Benevento M, Alpár A, Gundacker A, Afjehi L, Balueva K, Hevesi Z, et al. A brainstem-hypothalamus neuronal circuit reduces feeding upon heat exposure. Nature. 2024;628:826–34.
Sanders KM, Sakai K, Henry TD, Hashimoto T, Akiyama T. A subpopulation of amygdala neurons mediates the affective component of itch. J Neurosci. 2019;39:3345–56.
Su XY, Chen M, Yuan Y, Li Y, Guo SS, Luo HQ, et al. Central processing of itch in the midbrain reward center. Neuron. 2019;102:858–72.e5.
Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA²LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–414.
Satoh T, Yokozeki H, Murota H, Tokura Y, Kabashima K, Takamori K, et al. 2020 guidelines for the diagnosis and treatment of cutaneous pruritus. J Dermatol. 2021;48:e399–e413.
Kaur R, Sinha VR. Antidepressants as antipruritic agents: A review. Eur Neuropsychopharmacol. 2018;28:341–52.
Yu B, Shao Y, Zhang J, Dong XL, Liu WL, Yang H, et al. Polymorphisms in human histamine receptor H4 gene are associated with atopic dermatitis. Br J Dermatol. 2010;162:1038–43.
Schaper-Gerhardt K, Wohlert M, Mommert S, Kietzmann M, Werfel T, Gutzmer R. Stimulation of histamine H4 receptors increases the production of IL-9 in Th9 polarized cells. Br J Pharmacol. 2020;177:614–22.
Murata Y, Song M, Kikuchi H, Hisamichi K, Xu XL, Greenspan A, et al. Phase 2a, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of a H4 R-antagonist (JNJ-39758979) in Japanese adults with moderate atopic dermatitis. J Dermatol. 2015;42:129–39.
Werfel T, Layton G, Yeadon M, Whitlock L, Osterloh I, Jimenez P, et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:1830–7.e4.
Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F. A phase 3 trial of Difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382:222–32.
Deeks ED. Difelikefalin: first approval. Drugs. 2021;81:1937–44.
Wang Z, Jiang C, Yao H, Chen O, Rahman S, Gu Y, et al. Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition. Brain. 2021;144:665–81.
Munanairi A, Liu XY, Barry DM, Yang Q, Yin JB, Jin H, et al. Non-canonical opioid signaling inhibits itch transmission in the spinal cord of mice. Cell Rep. 2018;23:866–77.
Ádám D, Arany J, Tóth KF, Tóth BI, Szöllősi AG, Oláh A. Opioidergic signaling-A neglected, yet potentially important player in atopic dermatitis. Int J Mol Sci. 2022;23:4140.
Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–49.
Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–41.
Matsuda KM, Sharma D, Schonfeld AR, Kwatra SG. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619–25.e6.
Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Prim. 2018;4:1.
Nakahara T, Morimoto H, Murakami N, Furue M. Mechanistic insights into topical tacrolimus for the treatment of atopic dermatitis. Pediatr Allergy Immunol. 2018;29:233–8.
Ruzicka T, Bieber T, Schöpf E, Rubins A, Dobozy A, Bos JD, et al. A short-term trial of tacrolimus ointment for atopic dermatitis. European Tacrolimus Multicenter Atopic Dermatitis Study Group. N Engl J Med. 1997;337:816–21.
Saripalli YV, Gadzia JE, Belsito DV. Tacrolimus ointment 0.1% in the treatment of nickel-induced allergic contact dermatitis. J Am Acad Dermatol. 2003;49:477–82.
Touw CR, Hakkaart-Van Roijen L, Verboom P, Paul C, Rutten FF, Finlay AY. Quality of life and clinical outcome in psoriasis patients using intermittent cyclosporin. Br J Dermatol. 2001;144:967–72.
Grattan CE, O’Donnell BF, Francis DM, Niimi N, Barlow RJ, Seed PT, et al. Randomized double-blind study of cyclosporin in chronic ‘idiopathic’ urticaria. Br J Dermatol. 2000;143:365–72.
Zebda R, Paller AS. Phosphodiesterase 4 inhibitors. J Am Acad Dermatol. 2018;78:S43–s52.
Hashim PW, Chima M, Kim HJ, Bares J, Yao CJ, Singer G, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: A double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360–5.
Saeki H, Baba N, Oshiden K, Abe Y, Tsubouchi H. Phase 2, randomized, double-blind, placebo-controlled, 4-week study to evaluate the safety and efficacy of OPA- 15406 (difamilast), a new topical selective phosphodiesterase type-4 inhibitor, in Japanese pediatric patients aged 2-14 years with atopic dermatitis. J Dermatol. 2020;47:17–24.
Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927–40.
Ju T, Labib A, Vander Does A, Yosipovitch G. Topical Janus kinase-signal transducers and activators of transcription inhibitor tofacitinib is effective in reducing nonatopic dermatitis chronic itch: A case series. J Am Acad Dermatol. 2022;87:400–3.
Nakagawa H, Nemoto O, Igarashi A, Saeki H, Murata R, Kaino H, et al. Long-term safety and efficacy of delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with atopic dermatitis. J Dermatol. 2020;47:114–20.
Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kabashima K, Oda M, et al. Delgocitinib ointment in pediatric patients with atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J Am Acad Dermatol. 2021;85:854–62.
Kim BS, Howell MD, Sun K, Papp K, Nasir A, Kuligowski ME. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol. 2020;145:572–82.
Uppal SK, Kearns DG, Chat VS, Wu JJ. Ruxolitinib cream for the treatment of vitiligo. Lancet. 2020;396:1735–6.
Klaeschen AS, Wolf D, Brossart P, Bieber T, Wenzel J. JAK inhibitor ruxolitinib inhibits the expression of cytokines characteristic of cutaneous lupus erythematosus. Exp Dermatol. 2017;26:728–30.
Simpson EL, Lacour JP, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242–55.
Torrelo A, Rewerska B, Galimberti M, Paller A, Yang CY, Prakash A, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in paediatric patients with moderate-to-severe atopic dermatitis with an inadequate response to topical corticosteroids: results from a phase III, randomized, double-blind, placebo-controlled study (BREEZE-AD PEDS). Br J Dermatol. 2023;189:23–32.
Bieber T, Reich K, Paul C, Tsunemi Y, Augustin M, Lacour JP, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in patients with moderate-to-severe atopic dermatitis with inadequate response, intolerance or contraindication to ciclosporin: results from a randomized, placebo-controlled, phase III clinical trial (BREEZE-AD4). Br J Dermatol. 2022;187:338–52.
Zhang L, Guo L, Wang L, Jiang X. The efficacy and safety of tofacitinib, peficitinib, solcitinib, baricitinib, abrocitinib and deucravacitinib in plaque psoriasis – A network meta-analysis. J Eur Acad Dermatol Venereol. 2022;36:1937–46.
Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047–55.
Zhao Y, Zhang L, Ding Y, Tao X, Ji C, Dong X, et al. Efficacy and safety of SHR0302, a highly selective Janus Kinase 1 inhibitor, in patients with moderate to severe atopic dermatitis: a phase II randomized clinical trial. Am J Clin Dermatol. 2021;22:877–89.
Werth VP, Fleischmann R, Robern M, Touma Z, Tiamiyu I, Gurtovaya O, et al. Filgotinib or lanraplenib in moderate to severe cutaneous lupus erythematosus: a phase 2, randomized, double-blind, placebo-controlled study. Rheumatology. 2022;61:2413–23.
Landis MN, Arya M, Smith S, Draelos Z, Usdan L, Tarabar S, et al. Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: a phase IIb, randomized, double-blind, vehicle-controlled, dose-ranging and parallel-group study. Br J Dermatol. 2022;187:878–87.
Landis MN, Smith SR, Berstein G, Fetterly G, Ghosh P, Feng G, et al. Efficacy and safety of topical brepocitinib cream for mild-to-moderate chronic plaque psoriasis: a phase IIb randomized double-blind vehicle-controlled parallel-group study. Br J Dermatol. 2023;189:33–41.
Catlett IM, Hu Y, Gao L, Banerjee S, Gordon K, Krueger JG. Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. J Allergy Clin Immunol. 2022;149:2010–20.e8.
Strober B, Thaçi D, Sofen H, Kircik L, Gordon KB, Foley P, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40–51.
Lebwohl M, Warren RB, Sofen H, Imafuku S, Paul C, Szepietowski JC, et al. Deucravacitinib in plaque psoriasis: 2-year safety and efficacy results from the phase III POETYK trials. Br J Dermatol. 2024;190:668–79.
Armstrong AW, Gooderham M, Warren RB, Papp KA, Strober B, Thaçi D, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29–39.
Morand E, Pike M, Merrill JT, van Vollenhoven R, Werth VP, Hobar C, et al. Deucravacitinib, a tyrosine kinase 2 inhibitor, in systemic lupus erythematosus: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2023;75:242–52.
Tefferi A, Gangat N, Pardanani A. Jaktinib (JAK1/2 inhibitor): A momelotinib derivative with similar activity and optimized dosing schedule. Am J Hematol. 2022;97:1507–9.
Changelian P, Xu C, Mnich S, Hope H, Kostecki K, Hirsch J, et al. ATI-1777, a topical Jak1/3 inhibitor, may benefit atopic dermatitis without systemic drug exposure: results from preclinical development and phase 2a randomized control study ATI-1777-AD-201. JID Innov. 2024;4:100251.
Thoma G, Duthaler RO, Waelchli R, Hauchard A, Bruno S, Strittmatter-Keller U, et al. Discovery and characterization of the topical soft JAK inhibitor CEE321 for atopic dermatitis. J Med Chem. 2023;66:2161–8.
Deng L, Wan L, Liao T, Wang L, Wang J, Wu X, et al. Recent progress on tyrosine kinase 2 JH2 inhibitors. Int Immunopharmacol. 2023;121:110434.
Chen CX, Zhang W, Qu S, Xia F, Zhu Y, Chen B. A novel highly selective allosteric inhibitor of tyrosine kinase 2 (TYK2) can block inflammation- and autoimmune-related pathways. Cell Commun Signal. 2023;21:287.
Lleo A, Wang GQ, Gershwin ME, Hirschfield GM. Primary biliary cholangitis. Lancet. 2020;396:1915–26.
Nevens F, Trauner M, Manns MP. Primary biliary cholangitis as a roadmap for the development of novel treatments for cholestatic liver diseases(†). J Hepatol. 2023;78:430–41.
Younossi ZM, Stepanova M, Nader F, Loomba R, Anstee QM, Ratziu V, et al. Obeticholic acid impact on quality of life in patients with nonalcoholic steatohepatitis: REGENERATE 18-month interim analysis. Clin Gastroenterol Hepatol. 2022;20:2050–8.e12.
Marx D, Alnouri MW, Clemens S, Gedschold R, Riedel Y, Al Hamwi G, et al. Discovery of potent agonists for the predominant variant of the orphan MAS-related G protein-coupled receptor X4 (MRGPRX4). J Med Chem. 2023;66:15674–98.
Kotliar IB, Ceraudo E, Kemelmakher-Liben K, Oren DA, Lorenzen E, Dodig-Crnković T, et al. Itch receptor MRGPRX4 interacts with the receptor activity-modifying proteins. J Biol Chem. 2023;299:104664.
Ständer S, Yosipovitch G. Substance P and neurokinin 1 receptor are new targets for the treatment of chronic pruritus. Br J Dermatol. 2019;181:932–8.
Alam M, Buddenkotte J, Ahmad F, Steinhoff M. Neurokinin 1 receptor antagonists for pruritus. Drugs. 2021;81:621–34.
Welsh SE, Xiao C, Kaden AR, Brzezynski JL, Mohrman MA, Wang J, et al. Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: results from EPIONE, a randomized clinical trial. J Eur Acad Dermatol Venereol. 2021;35:e338–e340.
Vincenzi B, Trower M, Duggal A, Guglielmini P, Harris P, Jackson D, et al. Neurokinin-1 antagonist orvepitant for EGFRI-induced pruritus in patients with cancer: a randomised, placebo-controlled phase II trial. BMJ Open. 2020;10:e030114.
Azimi E, Reddy VB, Shade KC, Anthony RM, Talbot S, Pereira PJS, et al. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight. 2016;1:e89362.
Andoh T, Takayama Y, Kuraishi Y. Involvement of leukotriene B4 in dermatophyte-related itch in mice. Pharmacol Rep. 2014;66:699–703.
Pierre O, Fouchard M, Buscaglia P, Le Goux N, Leschiera R, Mignen O, et al. Calcium increase and substance P release induced by the neurotoxin Brevetoxin-1 in sensory neurons: involvement of PAR2 activation through both cathepsin S and canonical signaling. Cells. 2020;9:2704.
Braz JM, Dembo T, Charruyer A, Ghadially R, Fassett MS, Basbaum AI. Genetic priming of sensory neurons in mice that overexpress PAR2 enhances allergen responsiveness. Proc Natl Acad Sci USA. 2021;118:e2021386118.
Barr TP, Garzia C, Guha S, Fletcher EK, Nguyen N, Wieschhaus AJ, et al. PAR2 pepducin-based suppression of inflammation and itch in atopic dermatitis models. J Invest Dermatol. 2019;139:412–21.
Wilzopolski J, Kietzmann M, Mishra SK, Stark H, Bäumer W, Rossbach K. TRPV1 and TRPA1 channels are both involved downstream of histamine-induced itch. Biomolecules. 2021;11:1166.
Han Q, Liu D, Convertino M, Wang Z, Jiang C, Kim YH, et al. miRNA-711 binds and activates TRPA1 extracellularly to evoke acute and chronic pruritus. Neuron. 2018;99:449–63.e6.
Park CW, Kim BJ, Lee YW, Won C, Park CO, Chung BY, et al. Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: Phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). J Allergy Clin Immunol. 2022;149:1340–7.e4.
Gibson RA, Robertson J, Mistry H, McCallum S, Fernando D, Wyres M, et al. A randomised trial evaluating the effects of the TRPV1 antagonist SB705498 on pruritus induced by histamine, and cowhage challenge in healthy volunteers. PLoS One. 2014;9:e100610.
Dang TH, Kim JY, Kim HJ, Kim BJ, Kim WK, Nam JH. Alpha-mangostin: a potent inhibitor of TRPV3 and pro-inflammatory cytokine secretion in keratinocytes. Int J Mol Sci. 2023;24:12930.
Sun XY, Sun LL, Qi H, Gao Q, Wang GX, Wei NN, et al. Antipruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin. Mol Pharmacol. 2018;94:1164–73.
Neuberger A, Nadezhdin KD, Zakharian E, Sobolevsky AI. Structural mechanism of TRPV3 channel inhibition by the plant-derived coumarin osthole. EMBO Rep. 2021;22:e53233.
Qi H, Shi Y, Wu H, Niu C, Sun X, Wang K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm Sin B. 2022;12:723–34.
Neuberger A, Nadezhdin KD, Sobolevsky AI. Structural mechanism of TRPV3 channel inhibition by the anesthetic dyclonine. Nat Commun. 2022;13:2795.
Fan J, Hu L, Yue Z, Liao D, Guo F, Ke H, et al. Structural basis of TRPV3 inhibition by an antagonist. Nat Chem Biol. 2023;19:81–90.
Kittaka H, Yamanoi Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4) channel as a target of crotamiton and its bimodal effects. Pflug Arch. 2017;469:1313–23.
Qin Z, Xiang L, Zheng S, Zhao Y, Qin Y, Zhang L, et al. Vitexin inhibits pain and itch behavior via modulating TRPV4 activity in mice. Biomed Pharmacother. 2023;165:115101.
Jung MJ, Kim JC, Wei ET, Selescu T, Chung BY, Park CW, et al. A randomized, vehicle-controlled clinical trial of a synthetic TRPM8 agonist (Cryosim-1) gel for itch. J Am Acad Dermatol. 2021;84:869–71.
Kang SY, Choi MG, Wei ET, Selescu T, Lee SY, Kim JC, et al. TRPM8 agonist (cryosim-1) gel for scalp itch: a randomised, vehicle-controlled clinical trial. J Eur Acad Dermatol Venereol. 2022;36:e588–e589.
Lee S, Wei ET, Selescu T, Babes A, Park J, Kim J, et al. Histamine- and pruritogen-induced itch is inhibited by a TRPM8 agonist in humans: a randomized, vehicle-controlled trial. Br J Dermatol. 2024;190:885–94.
Tian W, He D, Liu J, Chen F, Zhang W, Hu J, et al. Topical borneol relieves nonhistaminergic pruritus via targeting TRPA1 and TRPM8 channels in peripheral nerve terminals of mice. Eur J Pharmacol. 2023;953:175833.
Luo M, He J, Yin L, Zhan P, Zhao Z, Xiong H, et al. Borneol exerts its antipruritic effects by inhibiting TRPA1 and activating TRPM8. J Ethnopharmacol. 2024;322:117581.
Bogacka J, Pawlik K, Ciapała K, Ciechanowska A, Mika J. CC chemokine receptor 4 (CCR4) as a possible new target for therapy. Int J Mol Sci. 2022;23:15638.
Matsuo K, Kitahata K, Kaibori Y, Arima Y, Iwama A, Ito M, et al. CCR4 involvement in the expansion of T helper type 17 cells in a mouse model of psoriasis. J Invest Dermatol. 2021;141:1985–94.
Sato M, Matsuo K, Susami Y, Yamashita A, Hayasaka H, Hara Y, et al. A CCR4 antagonist attenuates atopic dermatitis-like skin inflammation by inhibiting the recruitment and expansion of Th2 cells and Th17 cells. Int Immunol. 2023;35:437–46.
Kasamon YL, Chen H, de Claro RA, Nie L, Ye J, Blumenthal GM, et al. FDA approval summary: mogamulizumab-kpkc for mycosis fungoides and Sézary syndrome. Clin Cancer Res. 2019;25:7275–80.
Bissonnette R, DuBois J, Facheris P, Del Duca E, Kim M, Correa Da Rosa J, et al. Clinical and molecular effects of oral CCR4 antagonist RPT193 in atopic dermatitis: A Phase 1 study. Allergy. 2023;79:924–36.
Fu Y, Lin Q, Zhang Z, Zhang L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm Sin B. 2020;10:414–33.
Elsner JS, Carlsson M, Stougaard JK, Nygaard U, Buchner M, Fölster-Holst R, et al. The OX40 axis is associated with both systemic and local involvement in atopic dermatitis. Acta Derm Venereol. 2020;100:adv00099.
Iriki H, Takahashi H, Amagai M. Diverse role of OX40 on T cells as a therapeutic target for skin diseases. J Invest Dermatol. 2023;143:545–53.
Müller S, Maintz L, Bieber T. Treatment of atopic dermatitis: Recently approved drugs and advanced clinical development programs. Allergy. 2024;79:1501–15.
Guttman-Yassky E, Pavel AB, Zhou L, Estrada YD, Zhang N, Xu H, et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:482–93.e7.
Nakagawa H, Iizuka H, Nemoto O, Shimabe M, Furukawa Y, Kikuta N, et al. Safety, tolerability and efficacy of repeated intravenous infusions of KHK4083, a fully human anti-OX40 monoclonal antibody, in Japanese patients with moderate to severe atopic dermatitis. J Dermatol Sci. 2020;99:82–89.
Guttman-Yassky E, Simpson EL, Reich K, Kabashima K, Igawa K, Suzuki T, et al. An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: a multicentre, double-blind, placebo-controlled phase 2b study. Lancet. 2023;401:204–14.
Weidinger S, Bieber T, Cork MJ, Reich A, Wilson R, Quaratino S, et al. Safety and efficacy of amlitelimab, a fully human nondepleting, noncytotoxic anti-OX40 ligand monoclonal antibody, in atopic dermatitis: results of a phase IIa randomized placebo-controlled trial. Br J Dermatol. 2023;189:531–9.
Papp KA, Gooderham MJ, Girard G, Raman M, Strout V. Phase I randomized study of KHK4083, an anti-OX40 monoclonal antibody, in patients with mild to moderate plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1324–32.
Luo J, Zhu Z, Zhai Y, Zeng J, Li L, Wang D, et al. The role of TSLP in atopic dermatitis: from pathogenetic molecule to therapeutical target. Mediators Inflamm. 2023;2023:7697699.
Hoy SM. Tezepelumab: first approval. Drugs. 2022;82:461–8.
Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80:1013–21.
Dubin C, Del Duca E, Guttman-Yassky E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev Clin Immunol. 2021;17:835–52.
Kabashima K, Matsumura T, Komazaki H, Kawashima M. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: results from two phase III, long-term studies. Br J Dermatol. 2022;186:642–51.
Silverberg JI, Guttman-Yassky E, Thaçi D, Irvine AD, Stein Gold L, Blauvelt A, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. 2023;388:1080–91.
Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143:135–41.
Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101–12.
Liu T, Li S, Ying S, Tang S, Ding Y, Li Y, et al. The IL-23/IL-17 pathway in inflammatory skin diseases: from bench to bedside. Front Immunol. 2020;11:594735.
Krueger JG, Wharton KA Jr, Schlitt T, Suprun M, Torene RI, Jiang X, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144:750–63.
Mease PJ, Rahman P, Gottlieb AB, Kollmeier AP, Hsia EC, Xu XL, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1126–36.
Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397:754–66.
Mease PJ, Deodhar AA, van der Heijde D, Behrens F, Kivitz AJ, Neal J, et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022;81:815–22.
Xue X, De Leon-Tabaldo A, Luna-Roman R, Castro G, Albers M, Schoetens F, et al. Preclinical and clinical characterization of the RORγt inhibitor JNJ-61803534. Sci Rep. 2021;11:11066.
Nguyen E, Grajales-Reyes JG, Gereau RWT, Ross SE. Cell type-specific dissection of sensory pathways involved in descending modulation. Trends Neurosci. 2023;46:539–50.
|
Sources 2/ https://www.nature.com/articles/s41401-024-01400-x The mention sources can contact us to remove/changing this article |
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: [email protected]: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
to request, modification Contact us at Here or [email protected]



