Health
FDA Advisor Supports Novavax Covid-19 Vaccine for Approval
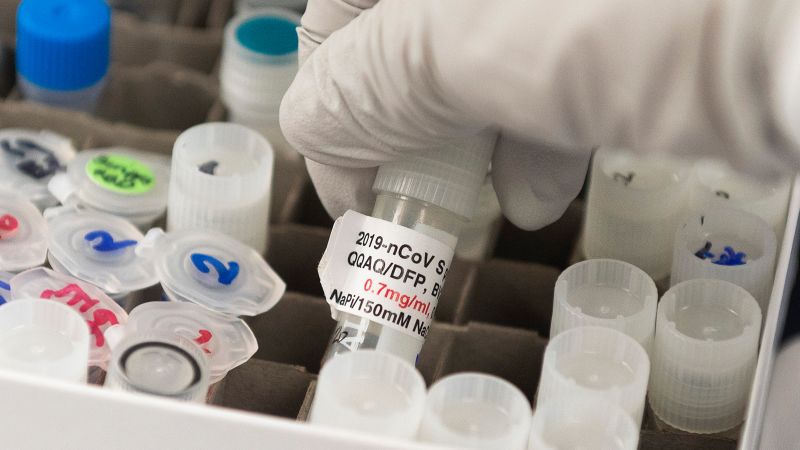

CNN
— —
The U.S. Food and Drug Administration’s Vaccine Advisor decided on Tuesday that it would be beneficial to authorize the Novavax Covid-19 vaccine for emergency use in adults, which uses a different technology than the three vaccines currently used in the United States. ..
Most of the FDA’s Vaccine and Related Biopharmaceutical Advisory Boards, based on available evidence, determine whether the benefits of the Novavax vaccine given as a primary series of two doses outweigh the risks of people over the age of 18. I answered the question and voted “yes”. Twenty-one members voted in favor, one abstained, and no one voted against.
When the full FDA gives a green light to the vaccine, it will be the fourth Covid-19 vaccine approved in the United States.
The Novavax vaccine is made using a small piece of coronavirus made in the laboratory to stimulate immunity. This protein-based approach is a more traditional approach to vaccine development than the Pfizer / BioNTech and Moderna mRNA vaccines.
Dr. Jay Portnoy, a committee member who is a professor of pediatrics at the University of Missouri’s Kansas City School of Medicine, said he saw the vote as an opportunity.
“This is another technology. It’s a more traditional protein-based vaccine,” Portnoy said. “Vaccines are given and deserve the opportunity to be studied.”
“Sure, the benefits outweigh the risks of the primary series,” said Dr. Michael Nelson, a committee member head of the Department of Asthma, Allergies and Clinical Immunology at the University of Virginia School of Medicine.
“This group is well aware that this is probably a three-dose series and that we need to accumulate data to support the need for booster and subsequent doses, perhaps in order to make a three-dose vaccine. I think it was, “he hinted. How will the debate about using vaccines as booster shots continue?
Prior to Tuesday’s meeting FDA Briefing Document Most side effects to the vaccine were mild to moderate and lasted only a few days, but myocarditis and pericarditis (inflammation of the heart muscle and inflammation of the tissues around the heart) occurred in 6 people after vaccination. discovered. Five of them developed inflammation within just two weeks of vaccination. For some, it happened 28 days later.
According to FDA documents, these cases resemble myocarditis after the mRNA Covid-19 vaccine developed by Pfizer / BioNTech and Moderna, causing “causal concerns” with the Novabax vaccine. I am.
Novavax statement There was no substantial difference in the incidence of myocarditis between vaccinated clinical trial participants (0.007%) and placebo-vaccinated participants (0.005%).
In November, Indonesia became the first country to grant an emergency use authorization for the Novavax vaccine. It is also licensed in the European Union, United Kingdom, Canada, South Korea, Australia, India, Philippines, New Zealand, etc.
In late January, Novavax I sent a request For the FDA to authorize a coronavirus vaccine for emergency use.
Novavax is ready to ship vaccine doses from the Serum Institute in India when approved by the FDA.
“Our hope is that the FDA will be able to receive this VRBPAC Commission’s recommendations along with all the manufacturing data we have provided and the inspection results of our Indian factories and reach a decision. Novavax President and Chief Executive Officer. The head, Stanley Erck, told CNN on Tuesday.
He said the company’s first shipments to the United States were “millions”.
“There are tens of millions of people who haven’t been vaccinated for some reason,” Elk said. “It’s one market for us.”
He added that other markets for vaccines include children and ultimately people who need booster immunity to maintain protection against Covid-19.
Novavax’s approval request was based on data containing the results of two large clinical trials showing an overall efficacy of approximately 90% and a “safety profile”. The study was conducted before the predominance of the Omicron coronavirus variant in the United States.
“The advisory board’s positive recommendations recognize the strength of our data and the importance of protein-based COVID-19 vaccines developed using an innovative approach to traditional vaccine technology.” Erck said in a post-meeting statement on Tuesday.
“At today’s VRBPAC conference, we’ve heard significant support for vaccines from doctors, healthcare organizations, and consumers who are eagerly looking for protein-based vaccine options,” he said. “I’m looking forward to the FDA’s decision.”
Overall, Covid-19 vaccination in the United States, especially booster dose uptake, is slow, and one FDA official said at a meeting on Tuesday that it was a “very serious” problem.
“There are very serious problems with vaccination in the United States. I feel that we must do what we can to make people more comfortable accepting these potentially life-saving medicines. That’s what it is, “said Dr. Peter Markes, director of the Biopharmaceutical Evaluation and Research Center.
At the meeting, Dr. Eric Rubin, Editor-in-Chief of the New England Journal of Medicine and a member of VRBPAC, asked why another Covid-19 vaccine was needed in the United States when the three were approved or approved. From Pfizer / BioNTech, Moderna, Johnson & Johnson’s Janssen.
“The Janssen vaccine is not currently used as a front-line vaccine like the mRNA vaccine, so there remains a vaccine problem for those who do not want to get the mRNA vaccine because of concerns,” Marks said. I am saying. For vaccines developed by Pfizer / BioNTech and Moderna.
“Having a protein-based alternative may be more comfortable for some people in terms of receiving vaccines,” Marks said.
More than two-thirds Although some of the US population has been fully vaccinated, at least in the first series, Novavax officials say they will seek approval for the vaccine for use as a booster dose. The first series.
Protein-based vaccines like Novavax work by making the body’s immune system aware of slightly modified parts of the targeted virus. In this case, it means a fragment of coronavirus spike protein.
When the gene sequence of the virus that causes Covid-19 was released, scientists around the world said, “Spike proteinOn that surface. ” These spikes form large protrusions that make the coronavirus look like a crown. “Corona” is Latin For the “crown”.
Novavax scientists have identified the gene for the peplomer and created a modified version of that gene. Researchers have cloned the gene into a baculovirus that infects insects. They then infected moth cells, especially Reeve’s turtle cells, and urged them to produce coronavirus spike proteins. These virus-like nanoparticles were harvested to make a vaccine for Novavax.
Overall, the vaccine relies on recombinant nanoparticle technology and a Novavax adjuvant called Matrix-M to stimulate the immune response and high levels of neutralizing antibodies.
“The overall idea of a vaccine is to show the immune system something that looks, tastes, and acts like a virus, except that it doesn’t get sick. So I made a spike protein. Puts it in particles like soap bubbles, which is the size of the virus, “said Dr. Gregory Glen, Head of Research and Development at Novavax. I told CNN last year..
“It’s not infectious. You don’t touch the coronavirus itself,” Glenn added. “Then given to people, they have a very focused immune response only to spikes-and the hallmark of our vaccine is that it gives a very strong immune response and has few side effects, And the dose is very low and the vaccine can be stored at normal refrigeration temperatures. ”
|
Sources 2/ https://www.cnn.com/2022/06/07/health/novavax-covid-vaccine-fda/index.html The mention sources can contact us to remove/changing this article |
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: [email protected]: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
to request, modification Contact us at Here or [email protected]



