Systemic lupus erythematosus (SLE) is a multisystem, chronic, inflammatory, relapsing, autoimmune disease with diverse clinical manifestations. One of the most harmful side effects of systemic lupus erythematosus is diffuse alveolar hemorrhage, first identified by Osler in 1904. [1]Although mortality from diffuse alveolar hemorrhage (DAH) has declined in recent generations, reported rates are still between 0% and 92% (average 50%). Diffuse alveolar hemorrhage causes acute hemoptysis, renal failure, respiratory failure, and the need for mechanical ventilation, sepsis, and thrombocytopenia, all of which are associated with increased risk of death. [2].
Neurological problems are common and common in SLE. Central nervous system (CNS) involvement is one of the most frequent side effects that can occur at any stage of SLE. Peripheral nervous system involvement in SLE is rare, but manifests as mononeuropathy multiple and distal symmetric axonal polyneuropathy [3]It is very rare to have either the classic form of Guillain-Barré syndrome (GBS) or acute inflammatory demyelinating polyneuropathy (AIDP). In our case, we present with severe diffuse alveolar hemorrhage and Guillain-Barre syndrome as manifestations of SLE in a 20-year-old woman who developed severe hypoxemia leading to mechanical ventilation.
A 20-year-old woman with no previous medical history presented to the emergency department with fever, 10 episodes of vomiting (coffee grounds), and 4 episodes of diarrhea (green, no melena). She had a history of lower abdominal pain, headache, ear pain, oral ulcers (lower lip), general weakness, and dysuria with episodes of epistaxis.
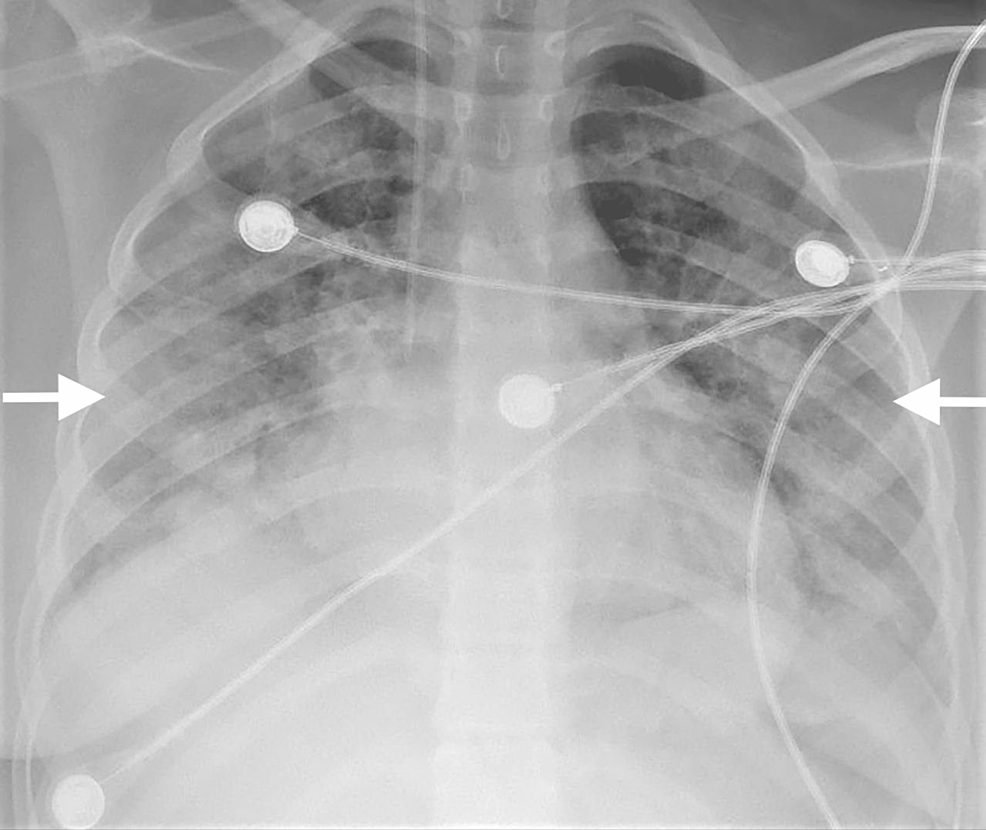
At presentation, the patient arrived in a hypoxic intensive care unit with high-flow oxygen. She had hypotension and was immediately intubated. She was started on low-dose norepinephrine infusions.A chest X-ray showed bilateral pulmonary infiltrates (Fig. 1).
shape
1: Chest X-ray showing bilateral pulmonary infiltrates.
Laboratory investigations revealed a hemoglobin of 6.39 g, platelets of 11,400,00, and a WBC of 15,800. Antinuclear antibody (ANA) and anti-dsDNA were positive with a titer of 1:1280 (fluorescence pattern, homogenous). Twenty-four hour urine protein showed a total protein of 17.778 g/day. Urinary creatinine was 13,900 μmol/L. C3 levels were <40 mg/dL and C4 <8 mg/dL. C-reactive protein was 96 mg/dL and all cultures were negative.
Echocardiography showed a left ventricular ejection fraction of approximately 52%, septal hypokinesis with mild tricuspid regurgitation, and a right ventricular systolic pressure of 24 mmHg. The patient underwent esophagogastroduodenoscopy, which revealed a congested gastric mucosa, and also hemorrhagic spots on the fundus.No vasculitis or features of vasculitis Helicobacter pylori Microorganisms were seen in the submitted biopsy. After 3 days of intubation, the patient was extubated, conscious and oriented.After the patient was stabilized, she underwent high-resolution computed tomography (HRCT), which showed bilateral diffuse alveolar hemorrhagic infiltrates (Fig. 2).
Chest computed tomography with contrast agent was performed, which revealed no radiographic evidence of pulmonary embolism. Therefore, echocardiography was repeated, revealing tachycardia, a 53% ejection fraction with midseptal hypokinesis, and mild mitral regurgitation suggestive of lupus myocarditis. Ultrasound abdomen was normal. Her oxygen demand was approximately 15 L/min, so she could not undergo a bronchoscopy. We decided to start her on pulsed methylprednisolone 1 g daily for 5 days. Because the response was weak, plasmapheresis was administered every other day for 6 doses, intravenous immunoglobulin (IVIG) 0.4 g/kg/day for a total of 5 doses, and intravenous cyclophosphamide 500 mg every 2 weeks for 6 doses. and extended pulse therapy. It took 7 days. After her one session of plasmapheresis, subsequent sessions were discontinued due to the effects of plasmapheresis on cyclophosphamide. The patient had lower extremity weakness, nerve conduction studies were performed, and there was marked reduction in motor amplification of all nerves examined, positive time dispersion, and absence of F-waves in the right ulna, acute motor axonal neuropathy ( AMAN) revealed preservation of sensory nerves with evidence of the type. of Guillain-Barré syndrome (GBS). The treatment regimen was cyclophosphamide Euro-Lupus 500 mg every 2 weeks for 6 doses, intravenous immunoglobulin (IVIG) for 5 days, and trimethoprim plus sulfamethoxazole 960 mg orally 3 times weekly. Changed. After her 15 days in the ICU, the patient showed dramatic improvement and was transferred to the ward, conscious and oriented. She had her vitals stabilized on a 2 L oxygen nasal cannula. On the ward, the patient underwent her second high-resolution computed tomography (HRCT) to rule out progression of alveolar hemorrhage, which showed a reduction in the volume and shape interval of the previous alveolar hemorrhage. I was.
She was discharged in a lethargic state. She continued follow-up with an OPD in Nephrology and Rheumatology to complete the last two doses of cyclophosphamide in the day care unit. 176 μmol/L and urine creatinine was shown to be 1015 μmol/L. Urinary protein random was 179 mg/L. Also, 0.16 mg/dL of c-reactive protein improved C3 levels to 104.6 mg/dL. She will continue to receive cyclophosphamide (one remaining dose).
Systemic lupus erythematosus (SLE) rarely develops the complication of diffuse alveolar hemorrhage (DAH).A rare early symptom of lupus [4]DAH in SLE is considered life-threatening, with a mortality rate of approximately 50%. [4]The pathophysiology of DAH includes pulmonary capillaritis, soft hemorrhage, and deposition of immune complexes on the alveolar walls, damaging the basement membrane and allowing red blood cells to escape into the alveolar space. [2]Lupus nephritis usually occurs within 3 to 5 years of the onset of SLE. Nephritis (~70% of cases) is the organ involvement most frequently associated with SLE cases with DAH. Lupus nephritis is primarily due to a type 3 hypersensitivity reaction that causes the formation of immune complexes. It binds to the mesangium along with anti-dsDNA. This causes an inflammatory response that leads to lupus nephritis.Anti-dsDNA was high in 75% of cases and complement was low in 86% of cases [5]More randomized clinical trials are needed to effectively treat DAH-associated SLE patients, and management is uniform across all medical centers. [6]Plasmapheresis, cyclophosphamide, and methylprednisolone are the most frequently used treatments. [7]Corticosteroids were cyclophosphamide (54%), plasmapheresis (31%), azathioprine (7%), intravenous immunoglobulin (IVIG, 5%), and mycophenolic acid (3%). , was used most frequently in one study involving 140 cases (98%). , B-cell targeted therapy rituximab (RTX, 6%), stem cell transplantation (2%) [5]The combination of methylprednisolone and cyclophosphamide used in various centers is associated with higher survival rates [8]Patients treated with cyclophosphamide showed better response and longer life compared to plasmapheresis [5].
Polyneuropathy of the peripheral nervous system is present in 10-20% of SLE cases [9]Nevertheless, GBS is a demyelinating polyneuropathy and a rare complication of lupus. [10]SLE with GBS occurs between 0.6% and 1.7%, indicating its rarity. [11]The first case of SLE with GBS symptoms was reported in 1964 [12]The diagnosis of Guillain-Barre syndrome in our case was based on electromyography showing limb symmetry, flaccid paralysis, and demyelinating polyneuropathy AMAN type. Our case met four American College of Rheumatology criteria for SLE: nephropathy (nephritis), anemia, positive ANA, and positive ds-DNA antibodies.
The etiology of GBS in SLE involves vascular occlusion of small vessels due to endothelial hyperplasia.Many anti-neural antibodies, such as anti-cardiolipin antibodies, anti-lymphocyte antibodies, lupus anticoagulants, damage the myelin component of nerves [13].
Neuropsychosystemic lupus erythematosus (NPSLE) is usually fatal in SLE patients due to an inflammatory pathway that causes immune complex formation and disrupts the blood-brain barrier. [14]Various treatments for GBS with SLE are recommended in the literature, including cyclophosphamide, corticosteroids, plasmapheresis, and immunoglobulins.Alone corticosteroid treatment did not produce good results [15].
For treatment, pulse therapy methylprednisolone 1000 mg IV once daily for 7 days, cyclophosphamide Euro-Lupus 500 mg every 2 weeks for 6 doses, and intravenous immunoglobulin (IVIG) 0.4 g/kg/day. Oral trimethoprim plus sulfamethoxazole 960 mg 3 times weekly for a total of 5 days. The patient is being followed up with her OPD in Rheumatology to complete a course of cyclophosphamide. During her follow-up, she is conscious, oriented, and fully mobile without assistive devices.
DAH and GBS are unpredictable and potentially fatal complications of SLE. Attention should be paid to the development of GBS and DAH, which can lead to high mortality if left untreated. Such patients should be treated early and aggressively with steroids, cyclophosphamide, and IVIG.