Health
Circulating myeloid-derived MMP8 in stress susceptibility and depression
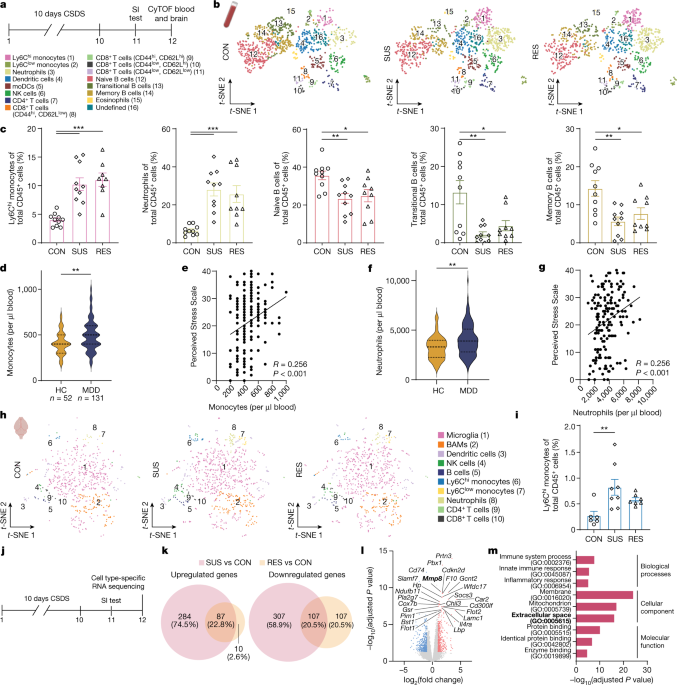
Mice
The following mouse strains were used: For standard CSDS experiments, 7-week-old C57BL/6 J (stock no. 000664) mice were purchased from The Jackson Laboratory. For bone marrow transplantation experiments, 4 week-old B6.SJL-Ptprca Pepcb/BoyJ (stock no. 002014, B6 CD45.1) mice were obtained from The Jackson Laboratory. B6.129(Cg)-Ccr2tm2.1Ifc/J (stock no. 017586, Ccr2rfp) and B6.129×1-Mmp8tm1Otin/J (stock no. 005514, Mmp8−/−) were bred inhouse. Four- to six-month-old male retired CD-1 breeders (Charles River Laboratories, Crl:CD1[ICR]) were used as aggressors for male CSDS. For the female CSDS experiment, male B6N.129S6(Cg)-Esr1tm1.1(cre)And/J (stock no. 017911, ERa–cre (ERa is also known as Esr1)) mice were purchased from The Jackson Laboratory and were crossed with CD-1 females to obtain F1 males, which were used as aggressors. Mice purchased from external vendors were allowed to habituate to the animal facility for at least one week. Mice were maintained on a 12 h light:dark cycle (lights on at 07:00, lights off at 19:00) with ad libitum access to food and water. For all behavioural tests, mice were allowed to acclimate to the testing room for at least 1 h. All procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the Icahn School of Medicine at Mount Sinai (ISMMS) Institutional Animal Care and Use Committee.
CSDS and SI test
For male CSDS19, retired male CD-1 breeders (age: 4–6 months) were used as aggressors. Before each defeat, aggressors were screened for aggressive behaviour for three consecutive days based on previously described criteria19. Two days before the start of the defeat, CD-1 mice were housed on one side of a perforated Plexiglass partition. During 10 consecutive days of CSDS, mice (7–8 weeks old) were subjected to direct physical interaction with a CD-1 for 10 min per day (5 min for bone marrow chimera cohorts), and the rest of the day placed on the other side of the Plexiglass divider, allowing for sensory but not direct physical contact. Male aggressors for female CSDS61,62 were generated as follows: Heterozygous ERa-cre mice were bilaterally injected with a Cre-dependent AAV-DIO-hM3D(Gq)-DREADD (Addgene, 44361-AAV2) into the ventrolateral subdivision of the ventromedial hypothalamus. To activate ERα+ cells, intraperitoneal injections of 1.0 mg kg−1 clozapine-N-oxide (Tocris, 4936) were administered 30 min before each defeat bout. Unstressed control mice were pair-housed across a Plexiglass partition. After the last day of defeat, stressed and unstressed control mice were singly housed (males) or kept in pairs (females). All stressed mice were carefully examined for wounding during the CSDS experiments and mice with excessive wounding were excluded.
Subthreshold stress
Subthreshold stress is a variation of the CSDS paradigm that is used to unravel pro-susceptible factors32 without eliciting behavioural alterations in unmanipulated mice. Experimental mice were exposed to 3× 5 min periods of direct physical interactions with an aggressive CD-1 mouse with a 15 min interval between defeats. 24 h after the last defeat bout, the SI test was conducted as described below.
SI test
The SI test was performed 24 h after the last defeat session under red-light conditions. After a 1 h habituation period to the behavioural suite, mice were placed into a Plexiglass arena (42 cm × 42 cm × 42 cm, Nationwide Plastics) with a small meshed enclosure on one end. For the first 2.5 min, the mouse freely explored the arena. The mouse was then removed from the arena which was subsequently cleaned with 70 % ethanol, then, a novel social target (CD-1 for males and ERa–cre for female CSDS) was placed into the enclosure and the mouse was placed back into the arena for another 2.5 min. Locomotor activity was tracked and recorded using a Noldus Ethovision System (Noldus Information Technology, version 11.0). SI ratio was calculated as the ratio between the time the mouse spent in the vicinity of the enclosure (SI zone) when a target mouse was present versus absent. Mice with an SI ratio of ≥1 show a behavioural profile similar to unstressed control mice and were termed resilient, while mice with an SI ratio <1 were termed susceptible. To test social avoidance behaviour towards a juvenile mouse, SI test was performed as described above with a four- to six-week-old male juvenile mouse as a social target. Additional parameters that were measured were total locomotion and time spent in corners, calculated as the sum between the two corners opposite the wire enclosure.
Chronic variable stress
Chronic variable stress was conducted in female mice as previously described63. For 21 days, mice were exposed to daily 1 h long stressors, consisting of either 100 mild foot shocks (0.45 mA), restraint stress in a 50 ml Falcon tube, or tail suspension. For the duration of the stress, mice were group housed.
Intraperitoneal injection of rMMP8
Before injection, rMMP8 (Bio-techne, 2904-MP-010) was activated ex vivo for 1 h at 37 °C with 1 mM 4-aminophenylmercuric acetate (APMA) in mercury-containing assay buffer (Anaspec, AS-71154) and then diluted in 0.9% sterile saline solution (VWR, 101448-952). For the dose–response experiment, we injected three different doses 50, 100 and 200 µg kg−1, and blood was drawn 20 min after the injection via submandibular bleeding and 18 h post-injection using trunk blood. MMP8 was measured as described below. For the behavioural experiments, mice were injected with 100 µg kg−1 rMMP8 or APMA 20 min before the defeat bout.
Stereotaxic surgeries, viral gene transfer or chronic hyaluronidase infusions
Surgeries were performed as described previously23. In brief, 6 week-old C57BL/6 J mice were injected intraperitoneally with a mixture of ketamine hydrochloride (100 mg kg−1 body weight) and xylazine (10 mg kg−1 body weigh). After anaesthesia was confirmed, mice were placed on a stereotaxic instrument (David Kopf Instruments). For the Cldn5 knockdown experiment, we bilaterally injected 0.5 μl of virus (1.0 × 1011 infectious units per ml) expressing either AAV2/9-shRNA or AAV2/9-shRNA-Cldn5 into the NAc (coordinates from bregma: AP + 1.5 mm; ML ± 0.5 mm; DV − 4.4 mm). After 2 weeks of recovery, mice received doxycycline (2 mg ml−1 in drinking water) for another 2 weeks. For the hyaluronidase infusion experiment, 27 G guide cannulae were inserted bilaterally into the NAc (from bregma: AP + 1.5 mm; ML ± 0.5 mm; DV − 4.4 mm) and fixed onto the skull using dental cement (Grip cement; Dentsply). After two weeks of recovery, daily infusions of 5 U of hyaluronidase (Sigma-Aldrich, H1136) or saline were completed once daily for 10 consecutive days. All compounds and viruses were infused at a rate of 0.1 μl min−1 and allowed to passively diffuse for 5 min before removing the needles.
Generation of bone marrow chimeras
Bone marrow chimeras were generated as described10,28. To ablate the peripheral immune system of the host mouse, 5-week-old male B6 CD45.1 mice were irradiated with a total of 11 Gy, delivered in two doses of 5.5 Gy, 3–4 h apart (X-rad 320 Irradiator (Precision X-Ray)). Haematopoietic progenitor cells were isolated from the femur/tibia of either Mmp8−/− or Mmp8+/+ male donor mice (12 weeks old). One hour after the second dose of irradiation, 1 × 106 cells were injected retro-orbitally in mice anaesthetized with isoflurane. Host mice were then allowed to recover for a total of six weeks. Mice received antibiotic treatment (0.2% in drinking water) (Neomycin trisulfate, N1876, Sigma) during the first three weeks of recovery. The level of chimerism was assessed using flow cytometry, comparing CD45.1 (host) (mouse anti-CD45.1-PE-Cyanine7, clone A20, Invitrogen, 25-0453-81) and CD45.2 (donor) (mouse anti-CD45.2-BV421, clone 104, BD Bioscience, 562895) leukocytes, and measuring MMP8 in plasma (Abcam, ab206982).
sCPP
sCPP was performed as described64. The experiment was done under red-light conditions after mice were habituated to the CPP room for 1 h. The CPP chamber (Med Associates) consisted of 3 different compartments: a neutral middle part, and two adjacent chambers, each with distinct floors (grid pattern) and walls. On the pre-test day, mice were allowed to explore all three chambers for 20 min and the time spent in each chamber was recorded. Based on these durations, mice were balanced to account for pre-test preferences. During the four consecutive conditioning days, mice were conditioned twice per day: In the morning, mice were placed in one chamber for 15 min with a novel, same-sex juvenile (4 to 5 weeks old) C57BL/6 J mouse (paired chamber). In the afternoon, the mouse was put in the empty opposite chamber for the same amount of time (unpaired chamber). On the testing day, mice were again allowed to freely explore all chambers for 20 min and the time spent in each chamber was automatically recorded (Med Associates).
Sucrose preference test
Sucrose preference test was performed to assess hedonic behaviour towards a sweet gustatory stimulus11. Mice were given access to two water bottles (50 ml conical tubes with sipper tops) for 24 h for habituation. Then, one water bottle was exchanged with a bottle containing 1% sucrose (Sigma, S0389) in drinking water. After 24 h, the bottle positions were swapped to prevent position bias. After another 24 h, sucrose preference was assessed as follows (based on weight of bottles): (sucrose (g)/total fluid (g)) × 100%.
Splash test
The splash test, a test performed to assess self-care behaviour, was conducted under red-light conditions as described previously11. In brief, after 1 h of habituation to the testing room, a 10% sucrose solution was gently sprayed onto the lower back of the mouse. Behaviour was recorded for 5 min, and time spent grooming was scored.
EPM test
The EPM was conducted to assess anxiety-like behaviours11. After 1 h of habituation to the testing room, mice were placed on an elevated cross-shaped maze for 5 min under red-light conditions. The four arms (two arms without and two arms with walls, each arm of the maze measuring 12 × 50 cm) were elevated 1 m above the floor. Behaviour was tracked using a Noldus Ethovision System (Noldus Information Technology, version 11.0). Parameters assessed included time spent in closed arms, open arms and in the
Mass cytometry
Blood was collected directly into fluorescence-activated cell sorting (FACS) buffer (DPBS (Thermo Fisher Scientific, 14190144) containing 0.5% bovine serum albumin (Sigma-Aldrich, A9647) and 2 mM EDTA (Invitrogen, AM9260G)). Cells were pelleted and red blood cells (RBCs) were lysed using RBC lysis buffers (BD, 555899). Immune cells of the brain were isolated as previously described25. In brief, mice were anaesthetized with 10% chloral hydrate and transcardially perfused with ice-cold PBS (0.1 M). Brains were extracted, leptomeninges carefully removed and the brains then cut into small pieces using scissors in a total of 3 ml digestion buffer (RPMI (Thermo Fisher Scientific, 11875093) with 2% fetal bovine serum (Thermo Fisher Scientific, A3840001), 2 mM HEPES (Corning, 25-060-CI) and 0.4 mg ml−1 collagenase D (Roche, 12352200)). The cell suspension was then incubated for 30 min at 37 °C. Digestion was stopped by adding EDTA (Invitrogen, AM9260G) to a final concentration of 5 mM. Using blunt 18 G needles (BD, 303129), the cell suspension was gently homogenized, and the homogenate was passed through a 70 μm strainer (pre-wet with PBS) (Miltenyi Biotec, 130-095-823). Cells were pelleted, resuspended in 30% Percoll (Millipore Sigma, GE17-0891-01) and centrifuged for 30 min at 23,500g without brakes at 4 °C. The myelin layer was aspirated and the middle layer containing leukocytes was transferred into a conical tube. Cells were then washed and stained for 30 min on ice with a mix of metal-conjugated antibodies (Supplementary Table 1). After antibody staining, cells were incubated with cisplatin for 5 min at room temperature as a viability dye to enable exclusion of dead cells. Cells were then fixed in PBS containing 1.6% formaldehyde and a 1:4,000 dilution of Ir nucleic acid intercalator to label all nucleated cells. Immediately prior to acquisition, cells were washed in PBS, then in distilled water, and finally resuspended in distilled water containing a 1/10 dilution of Equation 4 Element Calibration beads (Fluidigm, SKU 201078). After routine instrument tuning and optimization, the samples were acquired on a CyTOF2 Mass Cytometer equipped with a Super Sampler fluidics system (Victorian Airships). The acquisition rate was <500 events per second. The resulting FCS files were concatenated and normalized using a bead-based normalization algorithm in the CyTOF acquisition software and uploaded to Cytobank (https://mtsinai.cytobank.org/cytobank/; Cytobank, Menlo Park, CA, v7.0). FCS files were manually pre-gated for CD45+ events, excluding dead cells, doublets and DNA-negative debris (Extended Data Fig. 1a). Data analysis was performed with Clustergrammer, a web-based tool for visualizing and analysing high-dimensional data (https://github.com/ismms-himc/LegendScreen_CyTOF).
Fluorescence-activated cell sorting and bulk RNA sequencing of leukocyte subpopulations
For the mouse leukocyte subpopulation sequencing experiment, trunk blood was collected directly into FACS buffer. Samples were centrifuged and RBC lysis was performed (BD, 555899). After washing the cell pellet with ice-cold DPBS, Fc receptor blocking (rat anti-CD16/CD32, clone 2.4G2, BD Biosciences, 553141) was performed on ice for 30 min. Cells were pelleted and washed once. Leukocytes were then stained with the following antibodies (all at 1:400): rat anti-CD11b-PE-Cyanine7 (clone M1/70, BioLegend, 101215), rat anti-Ly6C-PerCP–Cy5.5 (clone HK1.4, BioLegend, 128027), rat anti-Ly6G-PE (clone 1A8, BioLegend, 127607), rat anti-B220-FITC (clone RA3-6B2, BioLegend, 103205) and rat anti-CD90.2-APC (clone 53-2.1, BioLegend, 140312) for 30 min on ice protected from light. After an additional wash, cells were sorted directly into Trizol (Themo Fisher Scientific, 15596026) by a BD FACSAria II cell sorter. Raw flow cytometry data were analysed using FlowJo software (FlowJo LLC, version 10.6.2). Samples were flash frozen on dry ice and stored at −80 °C. RNA was extracted using the RNeasy Micro Kit according to the manufacturer’s instructions (Qiagen, 74004). RNA quality, RNA integrity number (RIN) and RNA concentrations were assessed using Nanodrop (Thermo Fischer Scientific) and Bionalyzer (Agilent, 5067-1513). 500 pg of purified RNA was used for library preparation, which was performed using the SMARTer Stranded Total RNASeq Kit –2 – Pico Input Mammalian (Takara, 634413). Libraries were barcoded for multiplexing. Before sequencing, library quality and concentration were measured using Qubit Fluorometric Quantitation (Thermo Fisher). Libraries were sequenced (2 × 150 bp, paired-end reads configuration, v4 chemistry) on an Illumina HiSeq machine at a minimum of 30 million reads per sample. Sequencing was performed at Genewiz. Raw sequencing reads from the samples were mapped to mm10 using HISAT2 v2.1.065. Counts of reads mapping to genes were obtained using htseq-count v0.12.4 against Ensembl v90 annotation66. Differential expression analysis was done using DESeq2 v1.26.0 package67. The fold change threshold was set at 2 (that is, log2(fold change) > |1|). GO terms were determined using DAVID, version 6.868. Only GO terms with an adjusted P value < 0.05 (FDR) and an overall of > 5% (involved genes/total genes) were considered.
Fluorescence-activated cell sorting and flow cytometry of immune cells in leptomeninges, dura and choroid plexus
Leukocyte subpopulation frequencies from brain border regions were isolated as previously described69. Mice were anaesthetized with 10% chloral hydrate and transcardially perfused with ice-cold PBS (0.1 M). Leptomeninges, dura and choroid plexus were carefully dissected on ice. Meninges were digested in RPMI (Thermo Fisher Scientific, 11875093) with 1.4 U ml−1 Collagenase VIII (Sigma-Aldrich, C2139) and 1 mg ml−1 DNAse 1 (Thermo Fisher Scientific, EN0521) for 15 min at 37 °C. Digested dura and leptomeninges and undigested choroid plexus were passed through a 70 μm cell strainer (pre-wet with PBS) (Miltenyi Biotec, 130-095-823) into a 15 ml conical tube. Cells were pelleted (300g for 10 min at 4 °C) and washed once with ice-cold PBS. Cells were then resuspended in FACS buffer, Fc receptor binding was blocked (rat anti-CD16/CD32, clone 2.4G2, BD Biosciences, 553141) and cells were stained with a viability dye (Thermo Fisher Scientific, 65-0865-14) for 30 min. Cells were washed and stained with the following fluorophore-conjugated primary antibodies for 30 min on ice (all dilutions: 1:400): rat anti-CD11b–FITC (clone: M1/70, Invitrogen 11-0112-81), Armenian hamster anti-TCRβ-PerCP–Cy5.5 (clone: H57-597, Invitrogen, 45-5961-80), rat anti-Ly6C–APC (clone: HK1.4, Invitrogen, 17-5932-82), rat anti-Ly6G–eFluor 450 (clone: 1A8-Ly6g, Invitrogen, 48-9668-82), rat anti-CD19–PE (clone: IDE, BD Pharmingen, 553786), rat anti-CD45–PE–Cy7 (clone: 30-F11, BD Pharmingen, 552848). After an additional wash, cells were resuspended in FACS buffer and sorted by a BD FACSAria II using the 70 μm nozzle to sort cells into 1.5 ml Eppendorf tubes containing TRIzol LS with a sort speed of approximately 10,000 events per second. Raw flow cytometry data were analysed using FlowJo software (FlowJo, version 10.8.1).
FACS and single-cell RNA sequencing
Brain-trafficking monocytes and brain-resident myeloid cells were isolated based on previous published protocols70. Twenty-four hours after the SI test, mice were euthanized by injecting 10% chloral hydrate and perfused transcardially with ice-cold 0.1 M PBS (pH 7.4). Brains were rapidly dissected, leptomeninges carefully removed, and brains put in ice-cold PBS (for brain-trafficking monocyte RNA-sequencing experiment) or bilateral NAc tissue punches were obtained from 1 mm thick coronal slices using 1.2 mm punches (for resident myeloid cell RNA-sequencing experiment) (GE Healthcare Life Sciences, 1205×41). All the following steps were performed strictly on ice. For whole brains, tissue was cut into small pieces, for punches no shredding was needed. Tissue was then transferred to DPBS and homogenized with pestles (Sigma, D8938-1) in ice-cold PBS (20 stokes with pestle A, 20 stokes with pestle B). The cell suspension was then passed through a 70 μm cell strainer (pre-wet with PBS) (Miltenyi Biotec, 130-095-823) into a 15 ml conical tube. Cells were pelleted (300g for 5 min at 4 °C), resuspended in 10 ml of ice-cold 40% isotonic Percoll (Millipore Sigma, GE17-0891-01) (diluted in PBS) and centrifuged for 30 min at 500g at 4 °C with full acceleration and braking. The myelin layer was aspirated, then the cell pellet was washed with 10 ml of ice-cold PBS by centrifuging at 300g for 5 min at 4 °C. Cells were then resuspended in FACS buffer, Fc receptor binding was blocked (rat anti-CD16/CD32, clone 2.4G2, BD Biosciences, 553141) and then cells were stained with a viability dye (Thermo Fisher Scientific, 65-0865-14) for 30 min. Cells were washed and stained with a combination of the following fluorophore-conjugated primary antibodies: rat anti-CD45–BV510 (clone 30-F11, BioLegend, 103137), rat anti-CD11b–PerCP-Cyanine5.5 (clone M1/70, BioLegend, 101227), rat anti-Ly6C–APC-Cyanine7 (clone HK1.4, BioLegend, 128025), and rat anti-Ly6G–eFluor 450 (clone 1A8-Ly6g, Thermo Fisher Scientific, 48-9668-82) at a 1:400 dilution for 30 min on ice. After an additional wash, cells were sorted by a BD FACSAria II using the 70 μm nozzle to sort single cells into 96-well plates containing master mix (see below) with a sort speed of approximately 10,000 s−1. Raw flow cytometry data were analysed using FlowJo software (FlowJo LLC, version 10.6.2). All single-cell RNA-sequencing experiments were performed at the Single Cell Core Facility of the Sulzberger Columbia Genome Center, New York. Library preparation and RNA sequencing was performed as described previously71. In brief, cells were directly sorted into master mix, containing 1× Maxima Reverse Transcriptase Buffer (Thermo Fisher Scientific, EP0742), 40 U Maxima H Minus Reverse Transcriptase (Thermo Fisher Scientific, EP0751), 4 U SuperaseIN (Thermo Fisher Scientific, AM2694), 15% PEG (VWR, 97061-102), 1 μM TSO (Integrated DNA Technologies), and nuclease-free water. Template-switching reverse transcription was performed with adapter-linked oligo primers containing both cell- and molecule-specific barcodes (Supplementary Table 6). Excess primers were removed by adding 2 μl of Exonuclease I (Thermo Fisher Scientific, EN0581) mix to each well and incubated at 37 °C for 30 min, 85 °C for 15 min, 75 °C for 30 s. All wells were then pooled into a single 15 ml conical tube and cDNA was purified and concentrated with Dynabeads MyOne Silane beads (Thermo Fisher Scientific, 37002D). The cDNA was split into duplicate reactions of 25 μl cDNA, 25 μl of 2× HIFI HotStart Ready Mix (Kapa Biosystems, 07958927001), and 0.2 M SMART PCR Primer (Supplementary Table 6). PCR was performed as described above. cDNA was purified with AMPure XP beads (Beckman Coulter, A63880), visualized on an Agilent TapeStation and quantified with a Qubit II fluorometer (Thermo Fisher Scientific). Library preparation was performed using a modified protocol of the Nextera XT kit (Illumina, FC-131-1024), purified twice with AMPure XP beads (Beckman Coulter, A63880), and visualized and quantified as described above. Pooled, 3’-end libraries were sequenced on an Illumina NextSeq 500/550 apparatus. Reads were aligned to the mouse genome reference GRCm38 using STAR (version 2.5)72. Reads were assigned to cells and unique molecular identifiers73. The expression matrix for single-cell data was processed using the package Seurat v3.1.5 in R74. Features for which fewer than 3 cells were detected were removed, effectively excluding unexpressed features. Cells having at least 1,000 and at most 4,000 features were retained. Cells with more than 5% of reads mapping to mitochondrial genes were discarded. The NormalizeData function was used to log-normalize the dataset with a scale factor of 10,000. The top 2,000 most variable features across cells were found using the function FindVariableFeatures. The ScaleData function was applied to scale the dataset. The variable features were used to carry out dimensional reduction using principal components analysis. The optimal number of principal components to be used for dimensional reduction using UMAP was determined using ElbowPlot. FindNeighbors and FindClusters functions were utilized to construct a nearest neighbour graph and cluster cells in the dataset. UMAP was generated using the function DimPlot. The FindAllMarkers function was applied to determine markers for clusters in the UMAP plot. The FindMarkers function was used to carry out differential expression analysis for the three groups.
iDISCO+ staining, imaging and ClearMap analysis
Twenty-four hours after the SI test, Ccr2rfp+/− mice were injected with 10% chloral hydrate and transcardially perfused with ice-cold 0.1 M PBS followed by 4% paraformaldehyde (PFA) (Electron Microscopy Sciences, 15713 S). Intact brains were dissected out of the skull and post-fixed in 4% PFA in PBS at 4 °C for 18 h. Brains were then cleared and stained according to the iDISCO+ staining protocol (http://www.idisco.info). The primary rabbit anti-RFP antibody (Rockland, 600-401-379, 1:1,000) and the corresponding secondary antibody (donkey anti-rabbit IgG, Alexa Fluor 647, Thermo Fisher Scientific, A-31573, 1:1,000) were incubated with the brains for 7 days each at 37 °C. A LaVision light-sheet microscope with zoom body was used for sagittal half brain scanning with dynamic focus and a step size of 4 µm. Brain images were processed as previously described using ClearMap (version 1)31. RFP+ cells were quantified using the cell detection module, with cell detection parameters optimized and validated based on the intensity and shape parameters of the signal. The autofluorescence channel was aligned to the Allen Institute’s Common Coordinate Framework using the Elastix toolbox. Brain areas were collapsed into their parent regions prior to analyses.
Western blot
Bilateral NAc tissue punches were briefly thawed on ice and digested for 60 min at 37 °C in 20 U ml−1 chondroitinase ABC (Sigma-Aldrich, C3667) in 25 mM Tris-buffered saline (Thermo Fisher Scientific, BP2471) with protease inhibitors (Thermo Fisher Scientific, 1861281). Samples were immediately cooled on ice, centrifuged for 20 min at 21,000g at 4 °C and the supernatant was transferred to a new tube. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, 23227). Samples were flash frozen and stored at −80 °C. Total protein (20 μg) was separated by electrophoresis with a SDS–PAGE polyacrylamide gel (Bio-Rad, 4561034) and transferred to a PVDF membrane (Bio-Rad, 1704157). The membrane was blocked with 5 % non-fat dry milk (Bio-Rad, 1706404) in 0.1% Tween-20 (Sigma-Aldrich, P7949) in Tris-buffered saline (Thermo Fisher Scientific, BP2471, TBS-T) and incubated overnight with primary antibodies against aggrecan (1:1,000, Sigma-Aldrich, AB1031) or horseradish peroxidase (HRP) conjugated β-actin (1:2,000, Cell Signaling, 12262) in 5% non-fat dried milk (Sigma-Aldrich, A9647) in TBS-T. Membranes were then washed for 1 h with TBS-T and then incubated with secondary antibodies (1:10,000, anti-rabbit IgG HRP-linked, Cell Signaling, 7074) for 3 h at room temperature. After washing the membrane, visualization was performed using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, 32209) with an iBright CL1500 Imaging System (Thermo Fisher Scientific, A44114). Quantification was done with ImageJ (NIH, v1.53f51)75. Uncropped blots are available in Supplementary File 1.
Quantitative real-time PCR
RNA from fluorescence-activated cell-sorted monocytes was isolated using the RNeasy Micro Kit according to the manufacturer’s instructions (Qiagen, 74004). RNA quality and RNA concentrations were assessed using Nanodrop (Thermo Fischer Scientific). RNA was reversed transcribed to cDNA using qScript (QuantaBio, 95048-100) and the PCR reaction was performed using the SYBR Fast Advanced Master Mix system (Thermo Fisher Scientific, A46012) with the following primers (Integrated DNA Technologies): Mmp8 (Mm.PT.58.6942600, primer 1: AGGATCAGTGGAGTGAGAGAG; primer 2: CAAGGTATTGGAGGAGATGCTC); Gapdh (Mm.PT.39a.1, primer 1: GTGGAGTCATACTGGAACATGTAG; primer 2: AATGGTGAAGGTCGGTGTG). Gene expression analysis was done using the 2(–ΔΔCT) method76 and samples were normalized to the housekeeping gene Gapdh.
Ex vivo electrophysiology
Brains were rapidly extracted from isoflurane-anaesthetized mice, and coronal sections (250 µm) were sliced using a Compresstome (VF-210-0Z, Precisionary Instruments) in cold (0–4 °C) sucrose-based artificial cerebrospinal fluid (aCSF) containing: 87 mM NaCl (Sigma-Aldrich, S7653), 2.5 mM KCl (Sigma-Aldrich, P9333), 1.25 mM NaH2PO4 (Sigma-Aldrich, 71507), 4 mM MgCl2 (Sigma-Aldrich, M2670), 0.5 mM CaCl2 (Sigma-Aldrich, C8106), 23 mM NaHCO3 (Sigma-Aldrich, S6297), 75 mM sucrose (Sigma-Aldrich, S7903), 25 mM glucose (Sigma-Aldrich, G7021). After 60 min in aCSF at 32 °C for recovery, slices were kept in oxygenated (95% O2, 5% CO2) aCSF containing 130 mM NaCl (Sigma-Aldrich, S7653), 2.5 mM KCl (Sigma-Aldrich, P9333), 1.2 mM NaH2PO4 (Sigma-Aldrich, 71507), 2.4 mM CaCl2 (Sigma-Aldrich, C8106), 1.2 mM MgCl2 (Sigma-Aldrich, M2670), 23 mM NaHCO3 (Sigma-Aldrich, S6297), 11 mM glucose (Sigma-Aldrich, G7021) at room temperature for the rest of the day and individually transferred to a recording chamber continuously perfused at 2 to 3 ml min−1 with oxygenated aCSF. Patch pipettes (4–6 MΩ) were pulled from thin wall borosilicate glass using a micropipette puller (P-97, Sutter Instruments) and filled with a potassium gluconate-based intra-pipette solution containing: 116 mM KGlu (Sigma-Aldrich, P1847), 20 mM HEPES (Sigma-Aldrich, H3375), 0.5 mM EGTA (Sigma-Aldrich, E0396), 6 mM KCl (Sigma-Aldrich, P9333), 2 mM NaCl (Sigma-Aldrich, S7653), 4 mM ATP (Sigma-Aldrich, A9187), 0.3 mM GTP (Sigma-Aldrich, 51120) (pH adjusted to 7.2 and osmolarity to 290 mOsm). Cells were visualized using an upright microscope with an IR-DIC lens and illuminated with a white light source (Scientifica). Excitability was measured in current-clamp mode by injecting incremental steps of current (0–300 pA, +20 pA at each step). For recording of sEPSCs, NAc MSNs were recorded from in voltage-clamp mode at −70 mV. Whole-cell recordings were performed using a patch clamp amplifier (Axoclamp 200B, Molecular Devices) connected to a Digidata 1550 LowNoise acquisition system (Molecular Devices). Signals were low pass filtered (Bessel, 2 kHz) and collected at 10 kHz using the data acquisition software pClamp 11 (Molecular Devices). Electrophysiological recordings were extracted using Clampfit 11 (Molecular Devices) and analysed with R (version: 3.6.1, http://www.R-project.org). All groups were counterbalanced by days after CSDS. All recordings were performed while blinded to the conditions.
Human participants and processing of biospecimen
Study participants with MDD and healthy controls, as assessed according to SCID-577, were recruited through the Depression and Anxiety Center for Discovery and Treatment at the ISMMS. The ISMMS review board approved the study, and written informed consent was obtained from all participants prior to any study procedure. Participants were compensated for their time and effort. They provided demographic information and underwent a psychiatric evaluation using the SCID-5 conducted by trained study staff. Participants completed the Quick Inventory of Depressive Symptomatology-SR (QIDS-SR) to measure depressive symptom severity78. The Perceived Stress Scale27, a 10-item self-rating scale, was used to determine perceived stress levels. All participants underwent biochemistry and haematological laboratory testing, urine toxicology and pregnancy (if applicable) testing. At the time of enrolment, all participants were free of medications known to affect the immune system for at least two weeks. Participants were free of active infections or systemic illness. Subjects with concomitant unstable medical illnesses were excluded. Participants were free of current substances of abuse. On the day of blood draw, patients were fasted for at least 6 h. Blood was drawn into Vacutainer Gold Top 5 ml Silica Gel tubes (BD, 365968) for serum isolation, EDTA tubes (BD, 365975) to assess complete blood count and differential count (Sysmex XN-9100 Automated Hematology System) and into BD Vacutainer CPT tubes (BD, 362761) for the isolation of peripheral blood mononuclear cells (PBMCs). For serum, blood was allowed to clot for >30 min, then centrifuged at 1,300g for 15 min at 4 °C, then aliquoted and stored at −80 °C. PBMCs were isolated according to the manufacturer’s instructions and cryopreserved in liquid nitrogen. On the day of analysis, all PBMCs were carefully thawed in a water bath at 37 °C. Cells were pelleted (300g for 10 min at 4 °C) and washed once with ice-cold PBS. Cells were then resuspended in FACS buffer. Fc receptor binding was blocked using anti-CD16/32 (clone 2.4G2, Bio X Cell, BE0307) and cells were stained with a viability dye (Thermo Fisher Scientific, 65-0865-14) for 30 min. Cells were washed and stained with the following fluorophore-conjugated primary antibodies for 30 min on ice (all dilutions: 1:400): mouse anti-CD45–V500 (clone HI30, Fisher Scientific, BDB560779), mouse anti-CD19–PE–Cy7 (clone SJ25C1, Fisher Scientific, BDB560911), mouse anti-CD24–PE (clone ML5, Fisher Scientific, BDB560991), mouse anti-CD27–APC (clone L128), mouse anti-CD38 PerCP–Cy5.5 (clone HIT2, Fisher Scientific, BDB551400) and mouse anti-IgD–V450 (clone IA6-2, Fisher Scientific, BDB561309). Cells were washed, then resuspended in FACS buffer before acquisition on a BD LSRFortessa cell analyser (BD Biosciences). Flow cytometry data were acquired using FACS Diva software (BD, v.9). Data were analysed using FlowJo software (FlowJo LLC, version 10.8.1). Gating of B cell subtypes was performed as described79.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays were performed according to the manufacturer’s instructions (mouse MMP8: Abcam, ab206982; human MMP8: R&D Systems, DMP800B). For brain lysates, total protein was measured with the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, 23225). Plates were read on a SpectraMax 340PC384 microplate reader (Molecular Devices) and MMP8 or total protein levels were calculated from a serial dilution curve using SoftMax Pro 5 software (Molecular Devices).
Multiplex assays
Mouse plasma cytokines and chemokines were determined with the Milliplex MAP mouse cytokine/chemokine magnetic bead panel multiplex assay according to the manufacturer’s instructions (Millipore Sigma, MCYTOMAG-70K), and mouse MMP2, MMP3, proMMP9 and MMP12 were measured with Milliplex MAP Mouse MMP Magnetic Bead Panels 1 and 2 (Millipore Sigma, MMMP1MAG-79K, MMMP2MAG-79K).
Transmission electron microscopy and image analysis
Mice were injected with 10% chloral hydrate and transcardially perfused with 0.1 M sodium cacodylate buffer followed by ice-cold 2% PFA and post-fixed with 0.5% PFA at 4 °C. Tissue was sectioned on a vibratome, and freeze substitution and low temperature embedding of the specimens was performed as described80,81,82. Slices were cryoprotected by immersion in increasing concentrations of glycerol (from 10% to 30% in PBS) (v/v). Sections were plunged rapidly into liquid propane cooled by liquid nitrogen (−190 °C) in a Universal Cryofixation System KF80 (Reichert-Jung). The samples were immersed in 1.5% uranyl acetate dissolved in anhydrous methanol (−90 °C, 24 h) in a cryosubstitution AFS unit (Leica). The temperature was raised from −90 °C to −45 °C in steps of 4 °C/h. After washing with anhydrous methanol, the samples were infiltrated with Lowicryl HM20 resin (Electron Microscopy Sciences) at −45 °C. Polymerization with ultraviolet light (360 nm) was performed for 48 h at −45 °C, followed by 24 h at 0 °C. Ultrathin sections (80 nm) were cut with a diamond knife on a Leica UC7 ultramicrotome and mounted on 300 mesh copper grids using a Coat-Quick adhesive pen (Electron Microscopy Sciences). Images (n = 10 per mouse) were taken using a Hitachi 7700 electron microscope (Hitachi High-Technologies Corporation America) equipped with a XR81-B-M1-BT-FX, 8 Megapixel digital camera (Advanced Microscopy Techniques). Images were then imported into Adobe Photoshop (Adobe, 2022) and the ECS was manually scored using a computer tablet. Scoring was done by two independent investigators blinded to conditions. Images were then imported into ImageJ (v1.53f51)75 and the percentage of marked area/total area was calculated.
Immunohistochemistry and confocal microscopy
Mice were injected with 10% chloral hydrate and transcardially perfused with ice-cold 0.1 M PBS (pH 7.4) followed by ice-cold 4% PFA (Electron Microscopy Sciences, 15713 S). Intact brains were dissected out of the skull and post-fixed in 4 % PFA at 4 °C for 18 h. Brains were then cryoprotected in 30% sucrose (Sigma, S0389), frozen and sliced on a cryostat at 35 μm thickness. Sections were washed with PBS three times and incubated in blocking solution (3% normal donkey serum (Jackson Immuno Research, 017-000-121), 0.3% Triton X-100 (Sigma, T9284) in PBS) for 2 h. Sections were then incubated in primary antibodies (rat anti-CD31, 1:300, Biolegend, 102501; rabbit anti-RFP, 1:300, Rockland, 600-401-379; goat anti-RFP, 1:200, Rockland, 200-101-379; rabbit; rabbit anti-AQP4, 1:400, Thermo Fisher Scientific, PA5-85767) overnight at 4 °C. The next day, sections were washed in PBS with 0.3% Tween-20 (PBST (Sigma, P7949)) three times for 15 min each, then incubated with anti-rabbit-Cy2 and anti-rat-Cy5 secondary antibodies for 2 h (1:400, Jackson Immuno Research, 711-225-152 and 712-175-153, respectively). Sections were washed again three times with PBST. Slices were then mounted on slides, air-dried overnight, dehydrated, and coverslipped with DPX (Electron Microscopy Sciences, 13510). All slices were imaged using a Zeiss LSM 780 confocal microscope. 3D reconstruction was performed with the IMARIS software (Oxford Instruments, v9.9).
Biotinylation
Biotinylation of mouse rMMP8 (Bio-techne, 2904-MP-010) was performed using the EZ-Link Sulfo-NHS-Biotin kit according to the manufacturer’s instructions (Thermo Fisher Scientific, A39256). Biotinylated rMMP8 was separated from unbound biotin using Pierce C18 Spin Columns, 7 K MWCO, (Thermo Fisher Scientific, 89882), which recovers proteins and macromolecules larger than 7 kDa. Biotinylated rMMP8 was injected retro-orbitally into anaesthetized mice. After 2 h of circulation, mice were euthanized and perfused with ice-cold PBS followed by 4% PFA. Brain tissue processing and imaging was performed as described in the Immunohistochemistry and confocal microscopy section, with the following antibodies: Biotin was visualized using the Oregon Green 488 conjugate of NeutrAvidin biotin-binding protein (Thermo Fisher Scientific, A6374). Counterstaining was performed using rabbit anti-NeuN (1:500, Abcam, ab177487) and rat anti-CD31 (1:300, Biolegend, 102501).
Statistical analysis
Detailed statistical information for each experiment can be found in Supplementary Table 2. Unless described otherwise, statistical analyses were performed with GraphPad Prism software (GraphPad Software, version 9) or SPSS version 24 (IBM, SPSS). Outliers were identified using the Grubbs’ test and excluded from statistical analyses. Level of statistical significance was set at P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
|
Sources 2/ https://www.nature.com/articles/s41586-023-07015-2 The mention sources can contact us to remove/changing this article |
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: [email protected]: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
to request, modification Contact us at Here or [email protected]



