Health
The mini lungs and other organoids helping to beat COVID
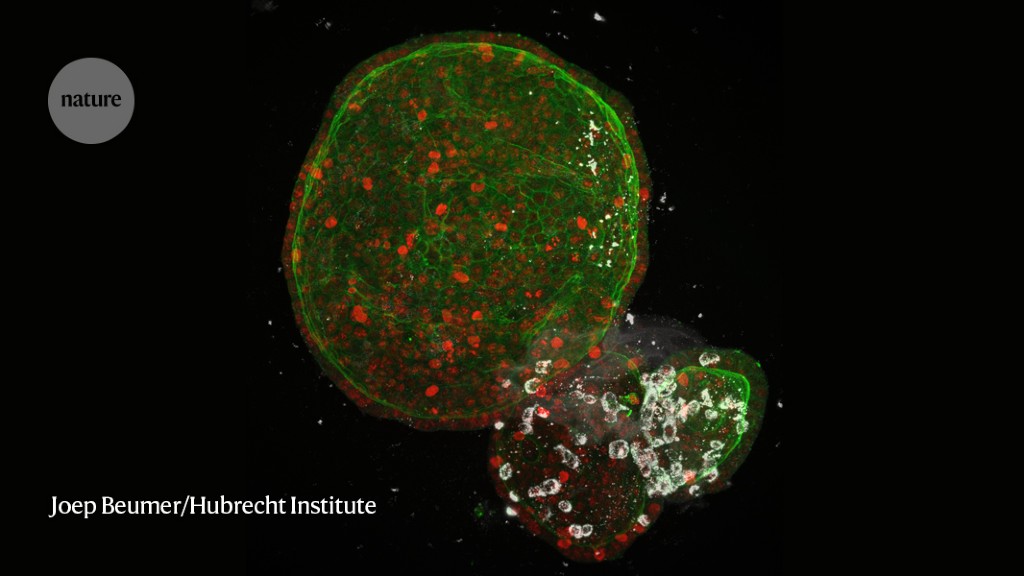
Shuibing Chen spent close to two months tending to her mini lungs — some half a million of them. Each one looked like a tiny storm cloud, ensconced in a warm dish and protected by a jelly-like dome. Chen, a stem-cell biologist at Weill Cornell Medicine in New York City, and her team had nurtured them from clumps of human cells, adding nutrients every few days as they grew into 3D air sacs.
These lung organoids matured until they reached the size of a lentil. Then, the team packed them up and transported them just a few blocks away, to a laboratory authorized to work with SARS-CoV-2, the virus responsible for the COVID-19 pandemic. There, the organoids were drowned in virus and each was doused with one of 15,000 drugs. Almost all of the mini lungs died, but a few of the drugs stemmed the infection — representing a handful of possible treatments for COVID-19.
Chen is one of many cell biologists who have been driven by the pandemic to push the boundaries of organoid technology for studying infectious diseases. In the past year, researchers have created mini lungs, guts, livers, brains and more to study how SARS-CoV-2 infects organs. They have learnt which cells the virus targets, the speed of that attack and how the cells retaliate.
“Organoids have found their way into the toolbox of virologists,” says Hans Clevers, a developmental biologist at the Hubrecht Institute in Utrecht, the Netherlands. The technology had previously been used mainly to study basic human biology, development and related disorders, and cancers, with only a few labs using the models to study viruses and other infectious diseases. But the pandemic has brought organoids to centre stage, spurring high-impact papers and demonstrating their value for drug development, says Clevers.
They are a welcome addition, because current methods of studying viruses have several limitations. The typical workhorse of virology is a cancerous cell line from the kidney of an African green monkey (Chlorocebus sabaeus), first extracted almost 60 years ago and dividing ever since. These cells, known as Vero cells, are excellent for growing viruses but don’t reflect the human body’s normal antiviral response. They are “really screwed up”, says Elke Mühlberger, a virologist at Boston University in Massachusetts. Researchers also use some cancerous human cell lines but, similar to the Vero cells, they don’t respond to infections in the way that normal cells would.
Although researchers have now established the potential relevance of organoids for studying new antiviral drugs, their work has not yet led to marketable treatments. “Organoid technology has benefited more from the pandemic than the treatment of COVID-19 has benefited from organoids, yet,” says Clevers.
To realize the technology’s full potential, scientists still need to find ways of growing more complex systems, for example by adding immune cells and blood vessels. Researchers also need to streamline the production process to create thousands, if not millions, of uniform organoids, quickly and cheaply.
“The use of organoids to study viruses is only at its infancy,” says Jie Zhou, a virologist at the University of Hong Kong.
Unculturable viruses
Before she started working with organoids, virologist Mary Estes relied on a much messier way to study the highly contagious vomiting bug norovirus. Nobody could grow the virus in the laboratory. So instead, she would isolate it from the excrement of people who willingly ingested it — and suffered the consequences — for the sake of her research.
In 2011, she saw a paper by Clevers in which he grew mini guts from stem cells scraped off the villi, the tiny tentacles that line people’s intestines1. Clevers had created the first organoids derived from adult stem cells, which grow almost indefinitely under the right conditions, and can build themselves into complex structures that reflect their organ of origin. (Organoids had already been made from embryonic stem cells or induced pluripotent stem (iPS) cells, which can develop into any cell type, but they typically reflect organs early in fetal development.)
“I thought — well, that looks like a system we ought to try,” says Estes, who is at Baylor College of Medicine in Houston, Texas. “Nobody else was using those cultures for virology at the time.”
In 2016 — almost half a century after its identification — Estes became the first person to grow human norovirus in a dish in a way that could be reproduced, in an intestinal organoid2.
Her studies proved that organoids were a good model of disease in people. She discovered, for instance, that norovirus variants did not replicate at all in organoids made from the cells of people who typically don’t get sick from the virus2.
Researchers have since used organoids to study many more viruses, including respiratory syncytial virus (RSV) — a common cause of lung infection in children — in airway organoids, and the rare and mysterious BK virus in kidney organoids.
In 2016, a team infected developing brain organoids with Zika virus and established a link between the infection in pregnant women and microcephaly3, a condition in which a fetus has an atypically small head. Ten days after being infected, the brain organoids were 40% smaller than the uninfected organoids. These neural progenitor cells are “fertile soil for Zika infection”, says Patricia Garcez, a neurobiologist at the Federal University of Rio de Janeiro in Brazil, who led the work.
And in 2018, Zhou, Clevers and their colleagues developed a lung organoid that could be used to rapidly assess the infectiousness of an emerging flu virus4. Strains known to be highly infectious in people, including the one that caused the 2009 H1N1 influenza pandemic, replicated much faster in the organoids than did strains that typically infect pigs and birds.
Aside from these examples, few virologists had experimented with organoids when SARS-CoV-2 emerged and grabbed their attention.
It didn’t take long for Clevers to recognize the potential of his organoid models for studying an unfamiliar virus in the middle of a pandemic. There were clear hints from the clinic that the virus could affect the gut, especially in children, says Clevers. He wondered whether he could use his intestinal organoids to see if the virus could infect gut tissue.
On 15 March 2020 — the day the Netherlands went into lockdown — he requested samples of SARS-CoV-2 from colleagues in Rotterdam. Within seven weeks, he and his colleagues published a paper in Science showing that SARS-CoV-2 readily replicated in mini guts, specifically targeting cells called enterocytes that line the intestines5. The study helped to explain why some people with COVID-19 have digestive problems, including diarrhoea and vomiting, and identified another possible route of transmission.
Researchers have since shown that SARS-CoV-2 can infect a host of mini organs, from the liver to kidneys to the brain — mimicking the multi-organ damage seen in some people with COVID-19.
The organ that has received the most attention — for good reason — is the lung. Buried deep in the lungs are tiny air sacs called alveoli — the site of pneumonia in people with severe COVID-19. These cells are difficult to access and study. Catherine Blish, a viral immunologist at Stanford University in California, and her colleagues turned to cells that spontaneously form these air sacs to investigate the infection.
The researchers found that the virus ran riot in organoids made from alveoli and from cells in the tiny airways that feed them6. In the alveoli, SARS-CoV-2 targeted the cells that cover the air-exposed surface, which are rich in the ACE2 receptor through which SARS-CoV-2 gains entry. The virus also affected cells in the airways that secrete a molecule for dealing with the constant stretching in the lungs, called club cells. “Without organoids, I don’t know that we would have discovered that club cells could support SARS-CoV-2 replication because nobody would have thought to put it on club cells,” says Blish.
Other studies in mini alveoli have revealed details of the battle that takes place between the virus and cells7. Young Seok Ju, a genome scientist at the Korea Advanced Institute of Science and Technology in Daejeon, found that it takes about a day for the cells to retaliate. A struggle ensues between the cells and the virus, and from day three, more than one-quarter of the cells begin to die.
Scientists also want to know more about how the virus gets into cells. In one study, researchers used the gene-editing technique CRISPR in gut organoids to identify two other proteins — TMPRSS2 and TMPRSS4 — that, together with ACE2, facilitate the virus’s entry8. Other labs are knocking out ACE2 entirely, to see whether the virus can still get in. “The more we study organoids, the more we realize that different types of cells use different mechanisms to support viral entry,” says Chen.
Organoids have also been used to study emerging variants of SARS-CoV-2. In one preprint, Clevers and his colleagues studied human airway, alveolar and intestinal organoids and found that the B.1.1.7 variant, first identified in the United Kingdom, could produce larger amounts of infectious virus at later stages of infection than could previously circulating variants9, which might explain why B.1.1.7 is more transmissible.
Tiny tonsils
A front-row view of the virus rattling through the body could help researchers to identify ways to stop it. Organoids help to fill the gap between watching the virus in cell lines, which lack the complexity of real tissue, and in animal models, which mirror human infection poorly and are expensive, says Arinjay Banerjee, a coronavirus researcher at the University of Saskatchewan in Canada, who plans on using intestinal organoids in his work.
Many drug candidates that look promising in sheets of cells tend to fail in later stages, says Blish. One striking example is hydroxychloroquine, which was among the first drugs touted as a treatment for COVID-19. Studies in Vero cells suggested that chloroquine could block the virus, but later clinical trials showed that it had no protective effect. Clevers and others looked at how SARS-CoV-2 infects intestinal organoids, and found that the route it used to access them was different from the route it used to infect Vero cells. They discovered that hydroxychloroquine could block the virus’s path in Vero cells but not in organoids10. “Had these original drug screens been done on organoids rather than on Vero cells, chloroquine would never have emerged as a promising candidate,” says Clevers.
Many research groups are trying to realize the potential of organoids for drug discovery. Chen has tested around 1,000 drugs on mini colons and mini lungs, and has identified seven that look promising11, including the antiviral drug remdesivir, which had already been shown to have some benefit for participants in clinical trials. Chen sees her results as proving the utility of organoid screening.
The 15,000-drug-screen in lung organoids was her largest attempt yet. She spent weeks tweaking her methods to create organoids that are as similar to each other as possible. “We always worry about the variation between organoids, and comparing apples to oranges,” says Chen. The screening is part of a larger project in which multiple labs are using different methods to study the same compounds, and comparing their results, she says.
Organoids have also been used to test vaccines. In January, researchers developed mini tonsils from snippets of discarded tissue taken during surgery. Tonsils play a key part in the body’s defence; they’re often the first organ to churn out immune cells against a pathogen to ensure long-lasting protection.
When researchers added a COVID-19 vaccine candidate, some of the tonsil organoids produced an immune reaction, generating killer T cells, as well as antibodies that could target the spike protein on the surface of the virus12. But much work needs to be done to understand whether what transpires in a dish reflects what will happen in the body.
Plus, organs in the body don’t exist in isolation. To really understand what happens when a person is infected with SARS-CoV-2, and whether therapies will work, researchers need more complex systems that include immune cells and blood-vessel cells.
In unpublished work, Takanori Takebe, a clinician scientist and stem-cell biologist at Tokyo Medical and Dental University, grew blood-vessel cells on liver organoids and found that smaller capillaries and veins are more susceptible to SARS-CoV-2 infection than are larger vessels. And Chen has grown immune cells called macrophages on sheets of cardiac muscle cells13, and is doing the same for her lung organoids. These experiments, together with studies in animal models, could help to resolve a continuing debate about what it is that makes COVID-19 so deadly — the virus itself, or a hyperactive immune response.
Ideally, researchers want to be able to link organoids together. These systems could reveal, for example, how an infection that starts in the lung influences the heart or gut. “The dream for every virologist would be to have different organs connected to each other,” says Mühlberger. “The closer we can get to the human organ, the better it will be, the more we will learn about why viruses are so pathogenic.”
In 2019, Takebe connected liver, bile duct and pancreas organoids14, but so far, no teams have published papers using multi-organ models to study SARS-CoV-2.
Next pandemic
The relationships formed between cell biologists and virologists will probably extend beyond COVID-19.
For every organoid Mühlberger has infected with SARS-CoV-2, she has run parallel experiments with Ebola virus, the cause of a deadly haemorrhagic fever for which there are very few models of infection. She has found that Ebola virus can infect almost every tissue, even reaching regions that SARS-CoV-2 cannot venture into. This ability could be what makes Ebola so deadly, she says.
As for predicting the next pandemic, some researchers are turning to organoids made from animal cells.
In early 2020, Zhou reached out to Shi Zheng-Li, a virologist at Wuhan Institute of Virology in China, who helped to identify the closest known relative of SARS-CoV-2 — the bat coronavirus RATG13. Shi said she had sequenced hundreds of coronaviruses from bats but had been able to grow only a handful of them. Zhou wondered whether she could help by growing organoids from bat tissue. These could be used to test drugs that can target a wide range of viruses with the potential to infect people.
Zhou nipped fragments of gut from wild horseshoe bats (Rhinolophus sinicus), and created miniature bat intestines made of multiple cell types. SARS-CoV-2 grew well on the intestines15 — the first evidence that the coronavirus could infect horseshoe bats, adding weight to the hypothesis that it originated in bats.
Studying viruses with organoids is still a new pursuit, but many consider them an exciting model for exploring interactions between human cells and viruses, and the technology could make the response to the next pandemic much faster, says Ju.
“These are magical cultures,” says Estes. “It’s just your imagination that limits where this field can go.”
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: [email protected]: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
Pictures Credit
to request, modification Contact us at Here or [email protected]








