Health
Non-invasive, continuous oral delivery of solid levodopa-carbidopa for management of Parkinson’s disease
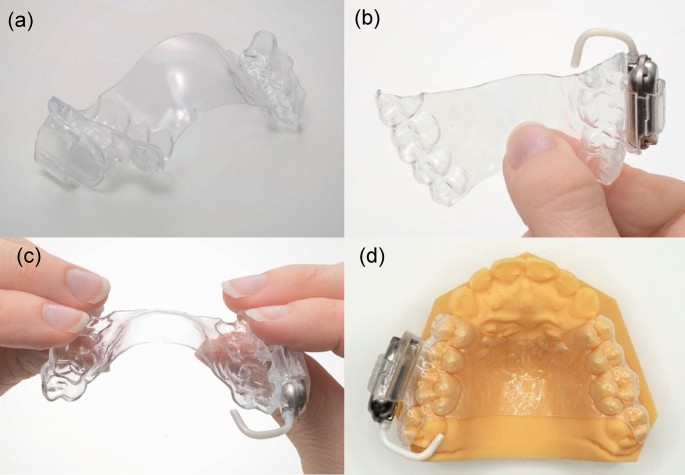
Design of the drug delivery system and the extruder
The drug delivery system, shown in Fig. 1, comprises a reusable, molded, copolyester orthodontic retainer with a co-molded pocket (Fig. 1a) into which the patient inserts a new, single-use, prefilled, disposable extruder (Fig. 1b and c). The titanium-walled extruder contains the below-described semisolid levodopa-carbidopa paste and a small volume of pressure-liquefied propellant in separate compartments. The paste extrudes at a constant rate. The patient replaces each extruder as it approaches depletion with a new extruder, typically after breakfast, lunch, and dinner. The system is removed for meals.
The drug delivery system. Photographs of (a) the orthodontic retainer having a co-molded pocket; (b-c) the retainer with the disposable extruder in its pocket; and (d) the retainer residing in the cheek pocket with its delivery tube delivering paste on the lingual side of the upper molars, where it is mixed with frequently swallowed saliva.
The retainer and extruder are not visible when worn and have no more impact on speech and swallowing than a standard orthodontic retainer. To avoid interference with speech and to minimize change in facial appearance, the co-molded copolyester pocket holding the extruder is in a buccal (cheek) pocket near an upper molar. The paste is, however, not extruded into the buccal cheek pocket, where it would stagnate for tens of minutes, but through a curved plastic delivery tube, to the lingual side of the molars (Fig. 1d). The paste has no taste. Mixed with saliva, the extruded and tasteless paste is swallowed frequently.
Photographs of the extruder are shown in Fig. 2. Materials selected for use in the construction of the extruder are restricted to materials with long histories of biocompatibility with the oral cavity. The case is made of 0.38 mm thick commercially pure titanium; being passivated by a film of titanium dioxide, the case does not corrode. The major drug paste containing chamber and the small propellant containing chamber are separated by a 30 µm thick deformable and impermeable silver diaphragm. The external surface of the plastic delivery tube is made of a medical-grade thermoplastic polyurethane (TPU).
Cross sectional views of the interior of the extruder are shown in the schematic drawings of Fig. 3. During manufacture, the drug paste chamber is filled with 1.04 g (0.84 mL of 1.24 g/mL density) paste through the fill port then plugged with an elastomeric fill plug. The propellant chamber comprises an elastomeric septum, through which 0.22 g (0.18 mL, 1.2 g/ml density) of pressure-liquefied 1,1,1,2-tetrafluoroethane (R-134a) is injected. The constant propellant vapor pressure causes deflection of the diaphragm, forcing constant extrusion of paste through two parallel flow controlling tubes and then into the delivery tube for delivery to the lingual side of the teeth. The plastic components, including the flow controlling tubes and internal surface of the delivery tube, are made of polyethylene terephthalate (PET). The delivery tube has an elongated elastomeric plug (not shown), which prevents delivery during storage and is removed by the patient to initiate flow.
The extrudable semisolid drug paste
The extrudable semisolid drug paste is engineered for safety, homogeneity, storage stability, and constancy of the extrusion rate. For safety, all paste components are restricted to common oral excipients and the established drugs LD and CD. The composition of the paste, formulated for years of storage without physical or chemical change, is shown in Table 1. The paste comprises 63% by weight of solid LD and solid CD, in a 4:1 ratio. Its five excipients, all listed on the FDA’s Inactive Ingredient Database and used in other FDA-approved orally-dosed drug products, are the edible oil Miglyol 812 N, a medium-chain triglyceride of 8-carbon caprylic and 10-carbon capric acids; water, the continuous phase of the suspension; Poloxamer 188, an emulsifying triblock polyethylene oxide-polypropylene oxide-polyethylene oxide copolymer, used in pharmaceuticals and cosmetics; antimicrobial benzoic acid; and the chelant and antioxidant edetate disodium (EDTA).
Development of unacceptable dose-rate changing physical heterogeneity upon storage (for example, by sedimentation of solid drug particles, by separation of the aqueous and oil phases, or by particle growth by Ostwald ripening) were prevented by the transport-slowing massive apparent viscosity (Fig. 4). Mixing of the components yielded an oil-in-water emulsion matrix, as demonstrated in Supplementary Fig. S1, providing for rapid dispersion of the drug paste in saliva. The emulsion is stabilized against coalescence and phase separation both by the surfactant and by the dispersal of micronized solid particles, as in a Pickering emulsion28.
Apparent viscosity of the drug paste. (a) Apparent shear rate dependence at 37 °C (red circles), 22 °C (green triangles), and 5 °C (blue squares), and the fit to a Herschel-Bulkley (‘H-B’) model (for the fitting parameters see Supplementary Table S1). (b) Temperature dependence at a shear rate of 5 s−1.
The paste is semisolid, exhibiting solid-like behavior below a defined apparent yield stress, and liquid-like behavior above that yield stress29. The dependence of the apparent viscosity on the shear rate is shown in Fig. 4a. In rheological terms, the paste is a Bingham pseudoplastic, meaning that above a certain yield stress, the apparent viscosity exhibits shear thinning. A fit to a Herschel-Bulkley model suggests a yield stress of 3–5.5 kPa at storage (5 °C), ambient (22 °C), and physiological (37 °C) temperatures (Fig. 4a and tabulated in Supplementary Table S1). Below these yield stresses, e.g., during storage, the paste exhibits solid-like behavior meaning near infinite viscosity. As experienced during oral extrusion, with apparent shear rates between 1 and 10 s−1 at 37 °C, the apparent viscosity is remarkably high, of magnitude 103 Pa s or 106 cP. The temperature dependence is relatively weak, with the apparent viscosity varying between 1200 and 1900 Pa s between 5 and 37 °C at a shear rate of 5 s−1 (Fig. 4b). The non-monotonic dependence on temperature is largely due to the Poloxamer’s well-known thermo-reversible gelling30. The physical stability, i.e., the absence of sedimentation or phase separation, is evident in Supplementary Fig. S2, showing that the paste withstands centrifugation at 22 °C at 4000g for 2.25 h (9000 g-hr), corresponding to storage at normal gravity for 1 year.
With both LD and CD being O2-oxidation-prone catechols, it is their rate of oxidation by dissolved O2 that controls the chemical stability. Being O2 diffusion-dependent, the bimolecular rate is inversely proportional to the viscosity, and is very slow at the near infinite apparent viscosity of the paste stored refrigerated (5 °C) or at ambient temperature (22 °C). Their oxidation is further slowed by the low solubilities of the drugs (1–10 mg/mL in water, nil in the oil), and by the low water concentration (< 7%w/w): less than 5 µmol of either LD or CD is dissolved in 1 g of paste. The stability is additionally improved by packaging in a laminated metal foil pouch. Stability is evident from the less than 20 ppm concentration of the downstream CD oxidation product hydrazine after two years of storage at 5 °C. Supporting chemical and physical stability data is tabulated in Supplementary Table S2.
Constant rate delivery
At the near-constant temperature of the mouth, the paste is extruded at a constant rate. The rheological data of Fig. 4 is used to characterize the drug paste flow behavior and design the flow controlling tubes of the extruder to provide the intended rate of paste extrusion. Due to the high yield stress of the paste and the slow rate of extrusion, the paste moves by plug flow, and the drug delivery rate is controlled primarily by wall slippage of the paste in the flow controlling tubes of the extruder31,32. Correspondingly, the delivery rate scales approximately with the cube of the internal radii of the flow controlling tubes and inversely with their lengths, as described in Supplementary Note 1. The flow controlling tubes can be tailored accordingly to provide the desired dose-rate by extending or trimming their length or by tuning their internal diameters.
In the case of plug flow, the wall slip velocity and delivery rate scale inversely with the formulation-dependent suspending fluid viscosity, as described in Supplementary Note 1, which depends strongly on the water content. The wall slip velocity scales with the slip layer thickness, which scales with the suspended particle size32, where larger particles provide for more wall-lubricating fluid in the particle-excluded volume near the wall. It depends on the material of the flow controlling tube, where, for example, surface roughening decreases the slip length31. In sum, for flow controlling tubes of fixed material and dimensions, the rate of paste slippage is dictated primarily by the wall-lubricating water content and secondarily by the solid particle size distribution. Both the water content and the particle size distribution are tightly controlled and closely monitored during manufacture and storage. For passage through the 0.3–0.4 mm internal diameter flow controlling tubes, the particles are micronized to a mean particle diameter of 2–10 μm and a maximum diameter of 30 µm, such that individual particles are not greater than 1/10th of the flow controlling tube diameter. No change in water content or particle size is observed during storage, as shown in Supplementary Table S2.
The photograph of Fig. 5 shows drug paste emerging from the tip of the delivery tube. Notably, the emerging semisolid paste retains its cylindrical shape and cross-sectional profile until it disperses in saliva.
The paste is extruded at a constant rate by constant-force deformation of the silver foil diaphragm upon volatilization of pressure liquefied 1,1,1,2-tetrafluoroethane confined to the propellant compartment. According to the Gibbs phase rule, the vapor pressure of a single component liquid is about constant at a constant temperature. In the mouth the temperature is about constant. Hot and cold drinks alter the temperature in the mouth only briefly, and as seen in Fig. 4b, the variation in apparent viscosity near 37 °C is mild, such that the amount of extruded paste over any 10-min period is unaffected.
The 0.38 mm thick titanium walls withstand the force exerted by the 8.4 bar pressure difference at 37 °C between the interior of the 1,1,1,2-tetrafluoroethane propellant chamber and the external sea-level atmospheric pressure. Each extruder can deliver at a constant rate approximately 80% or 800 mg of its paste, that is, each extruder can deliver about 400 mg LD and 100 mg CD at a constant rate. A patient using 3 extruders per day can therefore receive up to 1200 mg LD and 300 mg CD.
Figure 6 shows the constancy over 5 h of the paste extrusion rate for a random sample of n = 9 extruders from two separate product batches intended for 100 mg/hr paste delivery (50 mg/hr LD) and 136 mg/hr paste delivery (68 mg/hr LD). The group mean (± standard deviation) paste flow rates for the two batches are 100.5 ± 4.1 mg/hr and 136.0 ± 3.3 mg/hr, close to the targeted 100 mg/hr and 136 mg/hr, respectively. The chosen 5-hour period represents a typical interval between meals. The system is removed for meals and the extruder is replaced after breakfast, lunch, and dinner.
|
Sources 2/ https://www.nature.com/articles/s41598-024-78145-4 The mention sources can contact us to remove/changing this article |
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: [email protected]: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
to request, modification Contact us at Here or [email protected]









