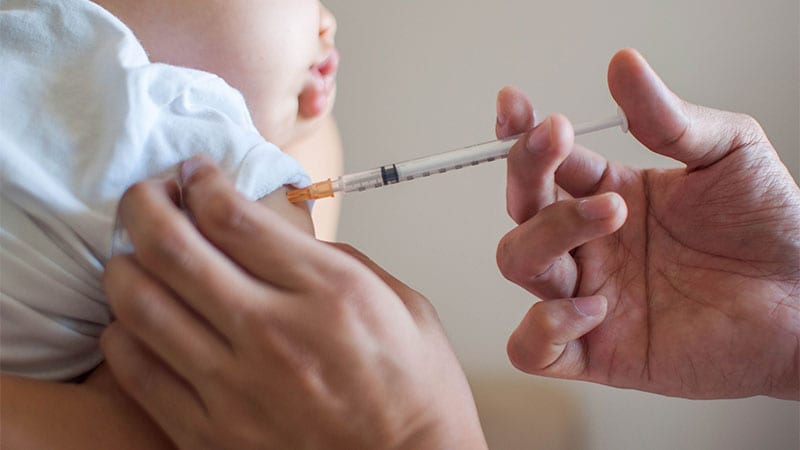
According to the results of Phase 3 trials, a single injection of the drug nilsevimab prior to the respiratory syncytial virus (RSV) season protects healthy infants from pathogen-related lower respiratory tract infections.
Previously published The study is a single dose Administration of nylsevimab was effective for preterm infants. Ability to protect all babies from RSV. Bronchiolitis Pneumonia, a major cause of hospitalization in this age group, “will be a paradigm shift in approach to this disease,” said William Mueller, a medical doctor at the Lully Children’s Hospital in Chicago and co-author of the study. rice field statement..
The primary endpoint of this study was medical participation in RSV-related lower respiratory tract infections. A single injection of nylsevimab was associated with a 74.5% reduction in such infections (P <.001), according to Mueller's group Published survey results March 2 New England Journal of Medicine..
Nilsevimab, a monoclonal antibody against the RSV fusion protein developed by AstraZeneca and Sanofi, has an extended half-life and can provide protection throughout the season with a single dose. The only approved option to prevent RSV, Palivizumab (Synagis) is used in high-risk babies and requires 5 injections to cover the viral season.
Nearly 1500 babies in more than 20 countries surveyed
To assess the efficacy of nilsevimab in late-term and full-term infants, researchers at 160 centers randomly assigned 1490 infants. Gestation period At least 35 weeks to receive an intramuscular injection of nylsevimab or placebo.
In 150 days after injection, 12 of the 994 infants who received nylsevimab had RSV-related lower respiratory tract infections that were medically involved, compared to 25 of the 496 infants who received placebo. It occurred (1.2% vs. 5%).
Six of the 994 babies who received nylsevimab were hospitalized for RSV-related lower respiratory tract infections, but eight of the 496 babies in the placebo group (0.6% vs. 1.6%). P = .07). The proportion of children hospitalized for respiratory illness as a result of RSV was 2.2% in patients receiving placebo compared to 0.9% in patients receiving nirsevimab.
Serious adverse events occurred in 6.8% of the nilsevimab group and 7.3% of the placebo group. None of these events, including the deaths of three people in the nilsevimab group, were considered to be associated with nilsevimab or placebo, according to the researchers. One infant who received nyrsevimab did not require treatment and suffered from a systemic, featureless, systemic macular rash that recovered in 20 days, they said.
Anti-drug antibodies were detected in 6.1% of the nilsevimab group and 1.1% of the placebo group. Researchers reported that these anti-drug antibodies tended to develop later and did not affect the pharmacokinetics of nilsevimab during the RSV season. It is not known how they affect subsequent doses of nylsevimab, they added.
In another report of the journal, researchers Dr. Joseph Domakowski, SUNY Upstate Medical College, Syracuse, NY, and colleagues said: Safety results From an ongoing study of nylsevimab, including infants Congenital heart diseaseChronic lung disease, and premature infants.
In this study, infants were given nirsevimab or palivizumab, and treatment appeared to have a similar safety profile, the authors reported.
Other approaches to RSV protection include passive antibodies obtained from vaccination of pregnant mothers and active vaccination of infants.
This publication was published last month by GlaxoSmithKline Suspend maternal RSV vaccine “We had the same goal of protecting infants from severe RSV infection,” said Louis Bont, MD, MD, Utrecht University Medical Center in the Netherlands.
RSV infection is one of the most deadly illnesses in infancy, and the Nilsevimab trial in more than 20 countries “changes the game,” Bont said. Medscape Medical News.. Still, researchers need to monitor RSV resistance to this treatment, he said.
Nilsevimab Reactive airway disease When asthma He said it was another open question.
“Finally, it should be remembered that RSV mortality is largely limited to developing countries, and it is unlikely that this new drug will be available in these countries in the coming years,” Bonn said. Said. “Nevertheless, nilsevimab can significantly reduce the overwhelming annual number of infants infected with RSV.”
Amy Sarah Ginsberg, MD, MD, of the University of Washington, Seattle, said Nilsevimab could benefit in low- and middle-income countries, including the possibility of being integrated into an established immunization program and given seasonally. He said he had sex. “But cost remains an important factor, as is the susceptibility to pathogen escape,” she said.
MedImmune / AstraZeneca and Sanofi funded the nirsevimab study. UMC Utrecht receives research grants and advice from AstraZeneca for Bonn’s RSV-related work.
N Engl J Med. Published online on March 2, 2022. Overview, correspondence
Follow Medscape for more news. Facebook, twitter, Instagram, YouTubeWhen LinkedIn