Health
Cute crying in Pink 1 reveals how kinase anchors to mitochondria
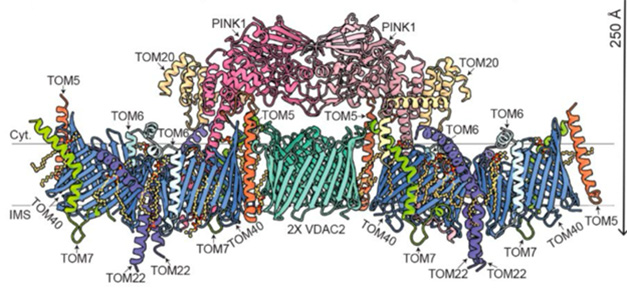
Parkinson's Disease Mystery – How Pink 1 is locked into mitochondrial malfunctions – has a structural solution. Researchers at the University of Melbourne led by Sylvie Calegari, Alisa Glucova and David Commander captured the first cryo-M snapshot of Human Pink 1, which was directly bound to depolarized mitochondria. Here, the kinase partners with TOM and VDAC to form an ultra-elevated complex that induces Pink 1 to the outer membrane. The study was published in Science on March 13th.
- Pink1 interacts with mitochondrial membrane gatekeeper.
- It forms a supercomplex with protein TOM and VDAC.
- The findings clarify the mechanism of stabilization of Pink1 membranes.
“This new Cryo-EM structure represents a remarkable advance in understanding how human Pink1 is stabilized.” “Commander and his team deserve high praise for this job.”
Pink1, a mutation, causes early-onset Parkinson's disease, ubiquitin kinase, acts as an early warning system around mitochondria (Valente et al. , 2004). Under normal conditions, when mitochondria maintain a delicate balance of the inside and outside of the bilayer, that is, the membrane potential, pink 1 is foamed into the outer layer and embedded inside. There, it is cleaved by the protease and the fragment is immediately sent to the cytosol for proteome treatment.
However, when the mitochondria loses charging, this routine stops and Pink 1 remains tied to the outer membrane. From this perch, the kinase domain of Pink1 acts to phosphorylate ubiquitin, which phosphorylates ubiquitin ligase parkin (another PD-binding protein), and tag mitochondria for degradation. The extensive strokes of this process are well known, but precisely, how Pink 1 remains anchored to the membrane of the diseased mitochondria was a puzzle.
To solve this, Karegari and his colleagues turned to high-resolution imaging. Using human embryonic kidney cells, scientists were transfected with tagged Pink1 and then treated the cells with oligomycin A. It is an ATP synthase inhibitor that depolarizes mitochondria and traps Pink1 on its surface. After lysing the cells to separate mitochondria, the researchers gently solubilized the membrane with detergent, pulling down pink1, and finally splitting these samples by size exclusion chromatography. Aut popped a 750 kDa supercomplex that contains not only multiple transcase components but also VDAC, acylglycerol kinases and a few chaperones.
By expanding this purification, scientists were able to accumulate enough protein in cryo-em. This revealed a spectacular assembly containing trans-lyxases of the outer membrane (TOM) and voltage-dependent anion channel (VDAC) that help to maintain mitochondrial membrane potential.
Two Tom core complexes with two copies of Tom40, Tom7, Tom6, Tom5 and Tom22, and a single Tom20 formed the backbone of the complex. These twin assemblies spanned a central VDAC2 dimer (TOM5) as molecular adhesive. Concealing the assembly, two Pink1 kinase domains are locked to the dimer and locked in place by interaction with Tom5 and Tom20. Meanwhile, the N-terminus of Pink1 was guided by Tom7 and Tom22, and emerged into the intermembrane space, meandering through the barrel-shaped Tom40 channel (image below). The TOM-VDAC assembly has never been documented before.

Mito Space Invader. A color-coded atomic model of the pink-TOM-VDAC complex as seen along the plane of the outer membrane. [Courtesy of Callegari et al., Science, 2025.]
“The structure is fantastic,” said Derek Narenda of the National Institutes of Health in Bethesda, Maryland. “Many of its features are surprising and will definitely reconstruct your understanding of damage sensing with Pink1” (comment below).

Pink1's perspective. Top-down views (left) and bottom-up views seen from the cytosol (left) as seen from the mesoramitochondrial membrane space (right). [Courtesy of Callegari et al., Science, 2025.]
In this structure, the disulfide bond of cysteine 166 locks together each pink 1 as a dimer (image below). This tandem arrangement reflects previous characterization of insect pink1 from the author's body lice (Gan et al. , 2022). This configuration is essential for the Pink1 dimers to phosphorylate each other. In lice, this step causes a conformational shift that forms the ubiquitin binding site. Interestingly, lice dimers must first be split into monomers to phosphorylate ubiquitin. This will recruit ubiquitin-ligase parkin. Pink1 attaches parkin to a phosphate base and activates its ligase activity. Picking up the baton, Parkin links the ubiquitin molecule to the damaged mitochondria and tagging it for degradation and recycling.

The kinase was discontinued. The atomic model of the Pink1 dimer (pink, fitting) is overlaid with the contours of the TOM-VDAC complex. Disulfide bonds shown as yellow spheres. The dimer is stable by binding in two Cys166s (in the box). [Courtesy of Callegari et al., Science, 2025.]
In human structure, researchers have discovered signs that the kinase domain has shifted to an activated state, indicating that autophosphorylation is already occurring. They suggest that their snapshots are locked in a “populated yet positive” conformation, namely a disulfide bond, and are not ready to phosphorylate ubiquitin.

Mito Mayday! 1. In healthy mitochondria, Pink1 is imported quickly into the inner membrane before being ingested and excreted. 2. you know! The membrane potential of falling mitochondria is stacked on membranes linked to Tom and VDAC to block the import of Pink1. We are ready for action. 3. Fires kinases: Pink 1 falls apart and phosphorylates ubiquitin molecules to membrane proteins such as VDACs. 4. Parkin joins: decorated with phosphobiquitin, VDAC draws with Parkin. Pink1 phosphorylates Parkin and activates it to stack more ubiquitin and signal transduction for mitochondrial cleanup. [Courtesy of Callegari et al., Science, 2025.]
What does this mean for Parkinson's disease? The authors hope that their structure paves the way for developing therapeutic activators that stabilize Pink1 in mitochondria. Muqit notes that the lack of structural information is hampering efforts to design small molecules that directly target Pink1. He believes this new structure will become a valuable resource. Abbvie is currently recruiting for a Phase I trial of small molecule Pink1 activators ABBV-1088.—George Heaton
George Heaton is a freelance author in Durham, North Carolina.
Paper quote
-
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Turkish D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-MaldonadoR, Der T, Salvi S, Cortelli P, Gilks WP, ha habve ratchmands, Auburger G, Wood NW.
Hereditary early-onset Parkinson's disease caused by mutations in Pink1.
Science. May 21, 2004; 304 (5674): 1158-60. EPUB 2004 April 15th
PubMed. -
Gan Zy, Callegari S, Cobbold SA, Cotton T, Mlodzianoski MJ, Schubert AF, Geoghegan ND, Rogers KL, Leis A, Dewson G, Glukhova A, Komander D.
Activation mechanism of Pink1.
Nature. February 2022; 602 (7896): 328-335. EPUB 2021 December 21st
PubMed.
External Quote
paper
-
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Turkish D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-MaldonadoR, Der T, Salvi S, Cortelli P, Gilks WP, ha habve ratchmands, Auburger G, Wood NW.
Hereditary early-onset Parkinson's disease caused by mutations in Pink1.
Science. May 21, 2004; 304 (5674): 1158-60. EPUB 2004 April 15th
PubMed. -
Gan Zy, Callegari S, Cobbold SA, Cotton T, Mlodzianoski MJ, Schubert AF, Geoghegan ND, Rogers KL, Leis A, Dewson G, Glukhova A, Komander D.
Activation mechanism of Pink1.
Nature. February 2022; 602 (7896): 328-335. EPUB 2021 December 21st
PubMed.
|
Sources 2/ https://www.alzforum.org/news/research-news/pretty-pink1-cryo-em-reveals-how-kinase-anchors-mitochondria The mention sources can contact us to remove/changing this article |
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: cgurgu@internetmarketingcompany.BizWebsite: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
to request, modification Contact us at Here or collaboration@support.exbulletin.com



