Health
NIH Begins Clinical Trial and Expands Supply of Intradermal Delivery of Monkeypox Vaccine – Global Biodefense
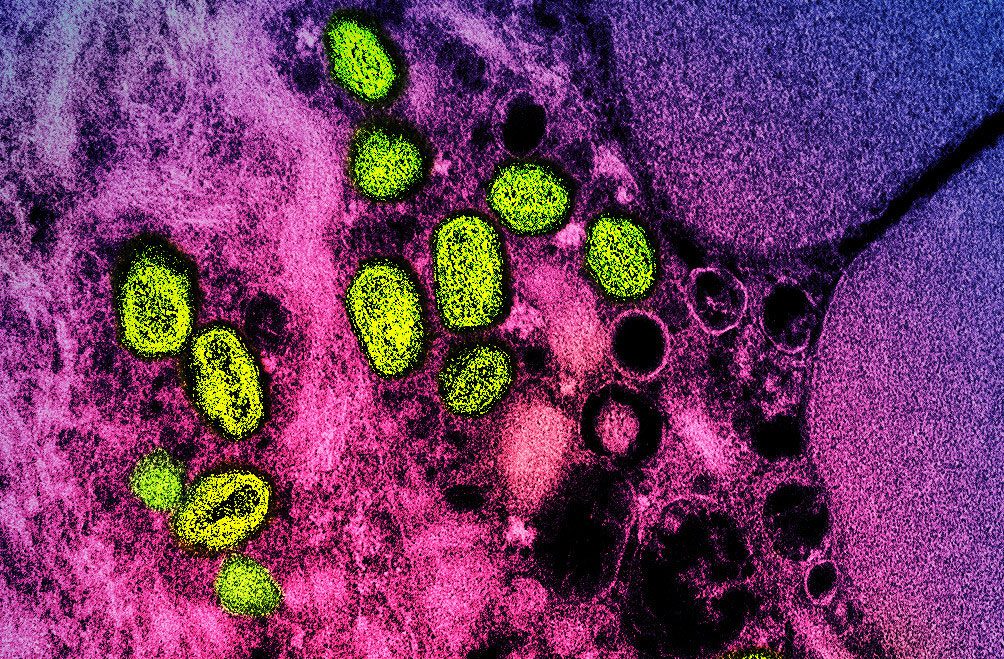
A clinical trial evaluating alternative strategies for administering the JYNNEOS monkeypox vaccine to increase the number of available doses has begun enrolling adult volunteers.
Adults between the ages of 18 and 50 who have never been vaccinated against smallpox or monkeypox are eligible to enroll in the NIAID trial. Investigators aim to include a demographically diverse group of volunteers representative of those affected by monkeypox.All trial participants will receive some form of her JYNNEOS vaccine. .
The trial, which will enroll more than 200 adults at eight research sites in the United States, is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. JYNNEOS is manufactured by Copenhagen-based Bavarian Nordic.
JYNNEOS contains an attenuated orthopoxvirus called Modified Vaccinia Ankara (MVA), which does not replicate in human cells. It is approved for administration by two subcutaneous injections (tissue under the skin) 28 days apart. However, the FDA recently approved the vaccine for intradermal (between layers of the skin) administration for adults. This alternative intradermal regimen uses one-fifth of the standard dose used for subcutaneous administration, allowing a healthcare provider to administer up to five times the vaccine dose per vial of his JYNNEOS vaccine. NIH-supported researchers Published in 2015 Intradermal administration of one-fifth the standard dose elicited an immune response in recipients comparable to subcutaneous administration.
“NIAID’s trial of JYNNEOS provides important information about the immunogenicity, safety, and tolerability of an alternative administration approach that expands the current supply of vaccines,” said Anthony S. Fauci, M.D., NIAID Director. said.
Since May 2022, the Centers for Disease Control and Prevention has reported 20,733 cases of monkeypox in the United States. The global pandemic is primarily affecting men who have sex with men. The virus usually causes painful skin lesions and flu-like symptoms. Serious complications that are rare in the United States include dehydration, bacterial infections, pneumonia, brain inflammation, sepsis, eye infections, and death. Historically, viruses have been known to be transmitted from person to person through direct contact with skin lesions, body fluids, respiratory droplets, and indirect contact with items such as contaminated clothing and bedding. I’m here. Preliminary analysis indicates that sexual transmission may play a role in the current outbreak.
Investigators should assess whether the peak immune response induced in recipients administered the vaccine intradermally is at least as great as that induced by the licensed subcutaneous regimen, and assess the relative to compare their overall safety and tolerability.
Volunteers will be asked to participate in eight research visits during the year. There, you will undergo a physical examination and be given a blood sample for laboratory evaluation. An independent Data and Safety Monitoring Board (DSMB) will monitor participant safety for the duration of the study.
This exam registers volunteers at:
- Saint Louis University of Missouri
- Baylor College of Medicine in Houston
- Brigham and Women’s Hospital in Boston
- NIH Clinical Center in Bethesda, Maryland
- George Washington University in Washington DC
- Vanderbilt University in Nashville, Tennessee
- Hope Clinic at Emory University in Decatur, Georgia
- University of California, San Diego
Investigators expect the trial to take 15 months to complete. However, the first results are likely for him to be available in early 2023.
A Trial to Evaluate the Immunogenicity of MVA-BN Monkeypox Vaccine Dose Reduction Strategy. ClinicalTrials.gov NCT05512949This study was a Phase 2, randomized, open-label, non-placebo-controlled, multi-site clinical trial to evaluate the efficacy of two intradermal modified vaccinia Ankara Bavarian Nordic (MVA-BN) vaccines compared to standard subcutaneous administration. (ID) To evaluate regimen (SC) regimen in healthy, vaccinia-naïve adults aged 18-50 years. At least 210 participants will be enrolled and randomly assigned to one of three study arms. Two dose-sparing strategies included 5-fold (2 x 10^7) and 1-fold (1 x 10^7) standard doses of MVA-BN administered ID on days 1 and 29. (arms 1 and 2 respectively). The comparison arm (arm 3) is a 2-dose standard (1 x 10^8) MVA-BN SC regimen.
The primary objectives were: 1) 2 x 10^7 50% tissue culture infectious dose (TCID50) peak humoral immune response following an ID regimen of MVA-BN to exceed the approved regimen of 1 x 10^8; to determine whether MVA-BN is not inferior to SC administration. 2) To determine whether the peak humoral immune response following an ID regimen of 1 x 10^7 TCID50 MVA-BN is not inferior to the approved SC dosing regimen of 1 x 10^8 MVA-BN. Secondary purposes are: 1) To determine whether peak individual humoral immune responses following each ID regimen are not inferior to approved regimens administered SC. 2) Assess the humoral immune response of each ID regimen (individually) compared to the licensed SC regimen on each study day. 3) To assess (separately) the kinetics of the humoral immune response of each ID regimen compared to SC regimens licensed up to day 365; 4) Systemic and local reactogenicity for 14 days after each vaccination; relative safety between study arms as assessed by sex, unsolicited adverse events during 28 days after each vaccination, and serious adverse events (SAEs) and medically-related events (MAAEs) from day 1. Up to day 57 for comparison and related SAEs/MAAEs up to day 181.
|
Sources 2/ https://globalbiodefense.com/2022/09/08/nih-begins-clinical-trial-of-intradermal-monkeypox-vaccine-delivery-to-expand-supply/ The mention sources can contact us to remove/changing this article |
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: [email protected]: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
to request, modification Contact us at Here or [email protected]



