Health
FDA signs off on updated Covid-19 vaccines that target circulating variants
CNN
—
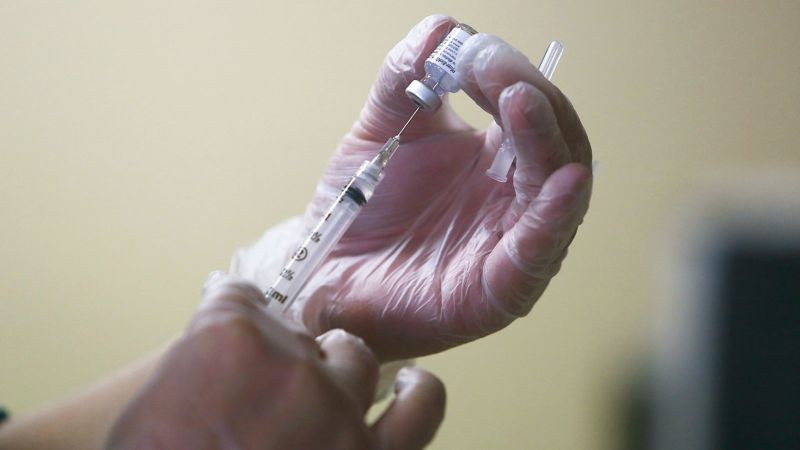
The US Food and Drug Administration gave the green light Monday to updated Covid-19 vaccines from Moderna and Pfizer/BioNTech amid rising cases and hospitalizations.
Both vaccine manufacturers have said testing shows that their vaccines are effective against EG.5, the currently dominant strain in the United States.
The Advisory Committee on Immunization Practices, a group of independent experts that advises the US Centers for Disease Control and Prevention on its vaccination decisions, will now weigh the safety and effectiveness of the updated vaccines and make recommendations for their use. After the CDC director signs off on those recommendations, the vaccines can be administered.
The advisory group is scheduled to meet to discuss Covid-19 vaccines Tuesday, meaning the vaccines could become available within just a few days at certain pharmacies and doctor’s offices.
Health officials are urging people to get vaccinated as soon as the shots are available. They’re debuting amid a late summer rise in Covid-19 hospitalizations in the United States and growing concerns about the effects that the triple threat of respiratory viruses – coronavirus, flu and respiratory syncytial virus – may have this fall and winter season.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a news release Monday. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
Experts have expressed concerns about the impacts the triple threat of viral illnesses will have this fall. The CDC has already warned that RSV levels are starting to pick up in the South, doubling between late July and late August. RSV cases are also beginning to slightly increase in parts of the West and Midwest, according to the CDC. However, the overall uptick is small relative to recent season peaks.
As for Covid-19, “at the present time, we are seeing an uptick in infection rates as well as hospitalizations, however, the absolute rates of severe disease, hospitalizations, and death are still very low compared with where we were a year ago and two years ago,” said Dr. Dan Barouch, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, who was not involved in developing either the Moderna or Pfizer vaccines but previously helped study the Johnson & Johnson vaccine.
For the approaching fall and winter, “the trajectory of where we’re going is difficult to tell. I don’t think we’re going to have a surge at the level that we saw a year or two years ago, but where it will peak I think remains to be seen,” he said. “Right now we have the vast majority of the population with vaccine immunity, natural immunity, or both. So there is a substantial level of population immunity already.”
Overall, the new FDA decision “comes at a time when COVID-19 cases are once again climbing. Now, most people 6 months or older in the U.S. are eligible to receive this season’s COVID-19 vaccine, even if they have never been vaccinated against COVID-19 before,” Albert Bourla, chairman and chief executive officer at Pfizer, said in a news release.
The updated vaccines are approved for people 12 and older and are authorized under emergency use for individuals 6 months through 11 years old. As part of the FDA’s update, the bivalent Moderna and Pfizer/BioNTech Covid-19 vaccines are no longer authorized for use in the United States.
According to the FDA, babies and young children ages 6 months through 4 years who have not been vaccinated against the coronavirus are eligible to receive three doses of the updated Pfizer/BioNTech shot or two doses of the updated Moderna booster.
For those in that age group who have been vaccinated, the number and timing of doses depends on the doses they’ve previously received.
According to the FDA, people 5 and older are eligible to receive a single dose of the updated vaccines at least two months since their last dose of any Covid-19 shot, regardless of previous vaccination.
“COVID-19 remains a leading cause of death in the U.S. and poses a significant threat to vulnerable populations, particularly as we enter peak respiratory virus season,” Stéphane Bancel, CEO of Moderna, said in a news release Monday.
“As the primary circulating strain continues to evolve, updated vaccines will be critical to protecting the population this season,” he said. “We appreciate the FDA’s timely review and encourage individuals who intend to get their flu shot to also get their updated COVID-19 vaccine at the same time.”
The benefit of the updated Covid-19 vaccines “will be greatest” in people at the highest risk of disease, Barouch said.
“The primary goal of these vaccines is to reduce the risk of severe disease, which is a very important goal, but it is not likely that these boosters will provide frank protection against the acquisition of infection and probably only modest effect on mild disease,” he said. “And so these boosters will likely be important for people who are at severe risk of disease.”
The mRNA vaccines have been updated to teach the body to fight the XBB.1.5 Omicron subvariant of the coronavirus and other closely related strains that are circulating.
Looking ahead, “barring the emergence of a markedly more virulent variant, the FDA anticipates that the composition of COVID-19 vaccines may need to be updated annually, as is done for the seasonal influenza vaccine,” the agency said Monday.
“Studies about confirmed viral infections suggest that COVID-19 adopts a seasonal pattern with peaks in fall and winter, similar to other respiratory viruses,” Dr. Ugur Sahin, CEO and co-founder of BioNTech, said in a news release. “Our goal is to provide people worldwide with COVID-19 vaccines that are adapted to circulating virus variants or sublineages.”
The updated Covid-19 vaccines, given in a single dose, “will be available to everyone who needs it, and it most likely will be available at no cost for the foreseeable future,” said Lori Tremmel Freeman, chief executive officer of the National Association of County and City Health Officials.
The FDA has not authorized an updated Covid-19 vaccine from Novavax, but the company said an updated version of its protein-based vaccine is currently under review by the FDA for authorization in people 12 and older.
Under the Affordable Care Act, most insurance plans are covering the full cost of vaccines, without co-pays. So most insured people will be able to get the updated Covid-19 vaccine at their doctor’s offices or pharmacies, such as CVS or Walgreens, at no cost.
People who are uninsured or underinsured may access the updated vaccine for free through the CDC’s Bridge Access Program. The new government program allows the CDC to purchase and distribute Covid-19 vaccines and allocate them through its network of state and local health departments. Vaccines for federally qualified health centers as well as certain pharmacy chains will be supported through both government and manufacturer-supplied resources.
The Bridge Access Program is temporary. According to the CDC’s website, free vaccines through the program will not be available after December 2024.
Although vaccines were previously provided for free by the government, this is the first time they will be provided through the commercial market. During a Pfizer investor call in October, officials said a potential US list price for the updated vaccine could be between $110 and $130 per single dose for adults.
CNN’s Brenda Goodman and Deidre McPhillips contributed to this report.
|
Sources 2/ https://www.cnn.com/2023/09/11/health/fda-signs-off-on-updated-covid-19-vaccines/index.html The mention sources can contact us to remove/changing this article |
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: [email protected]: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
to request, modification Contact us at Here or [email protected]



