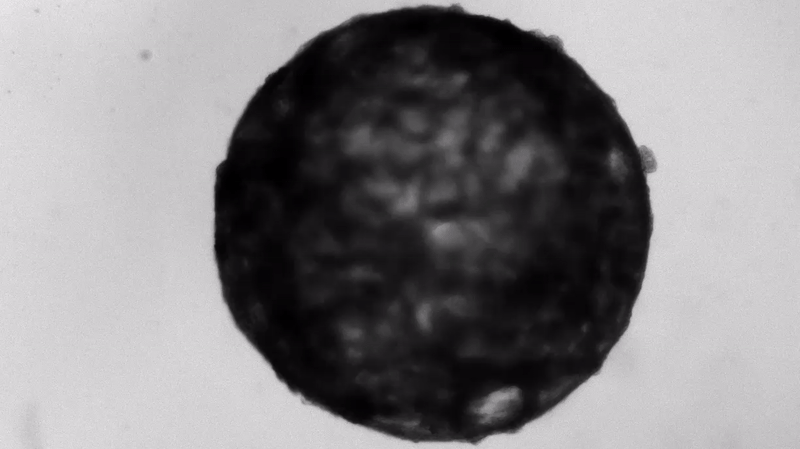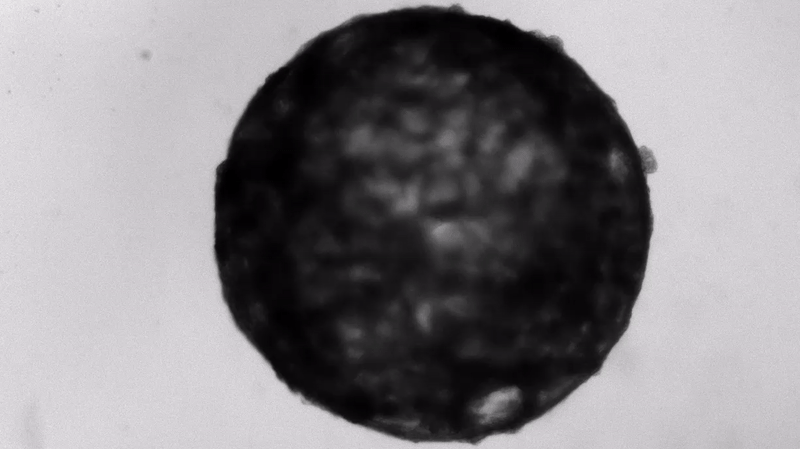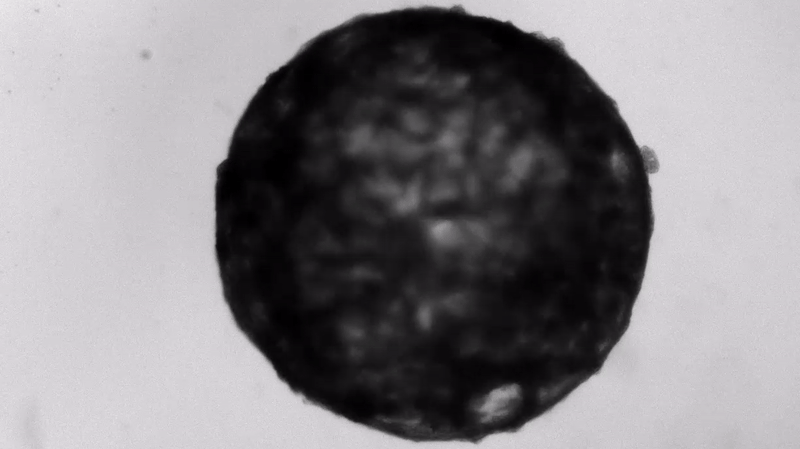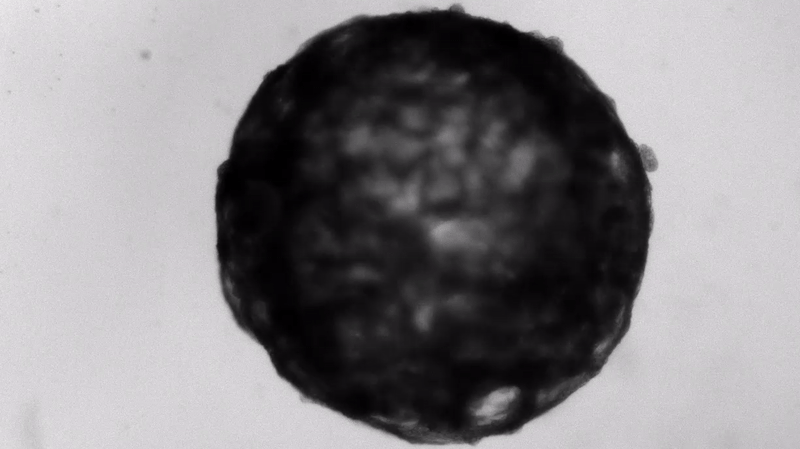
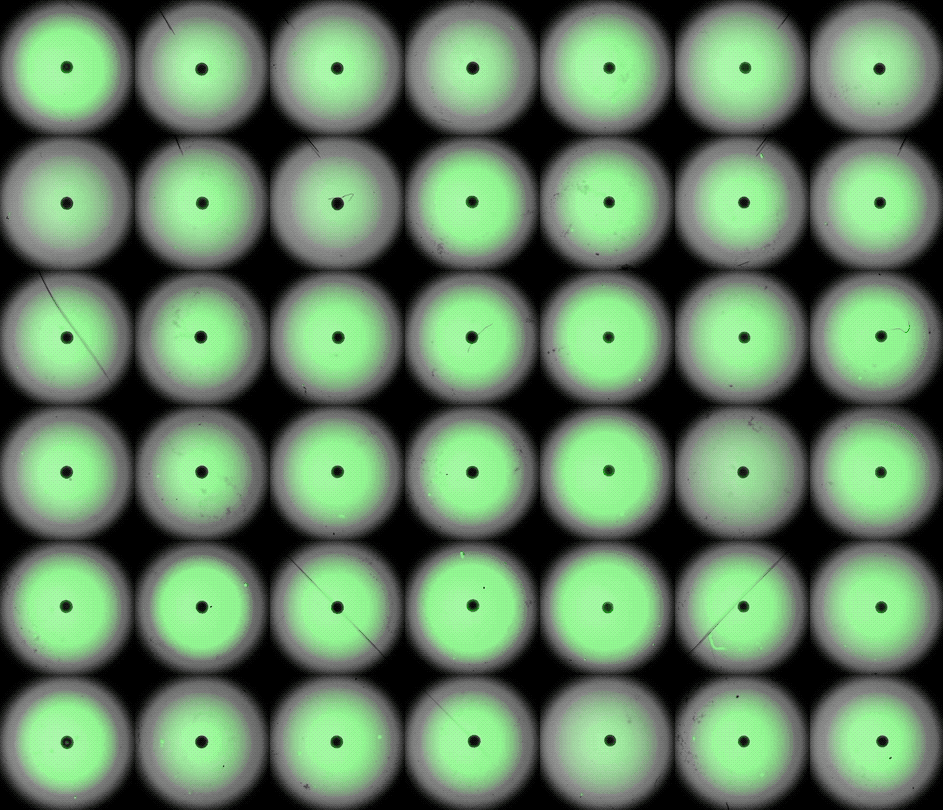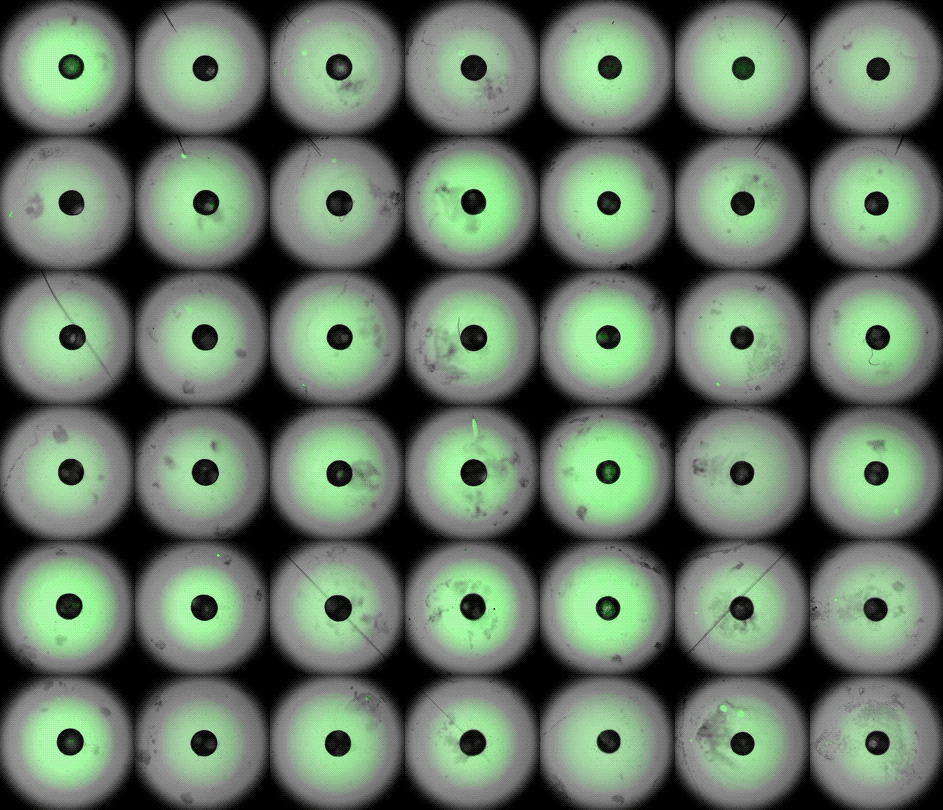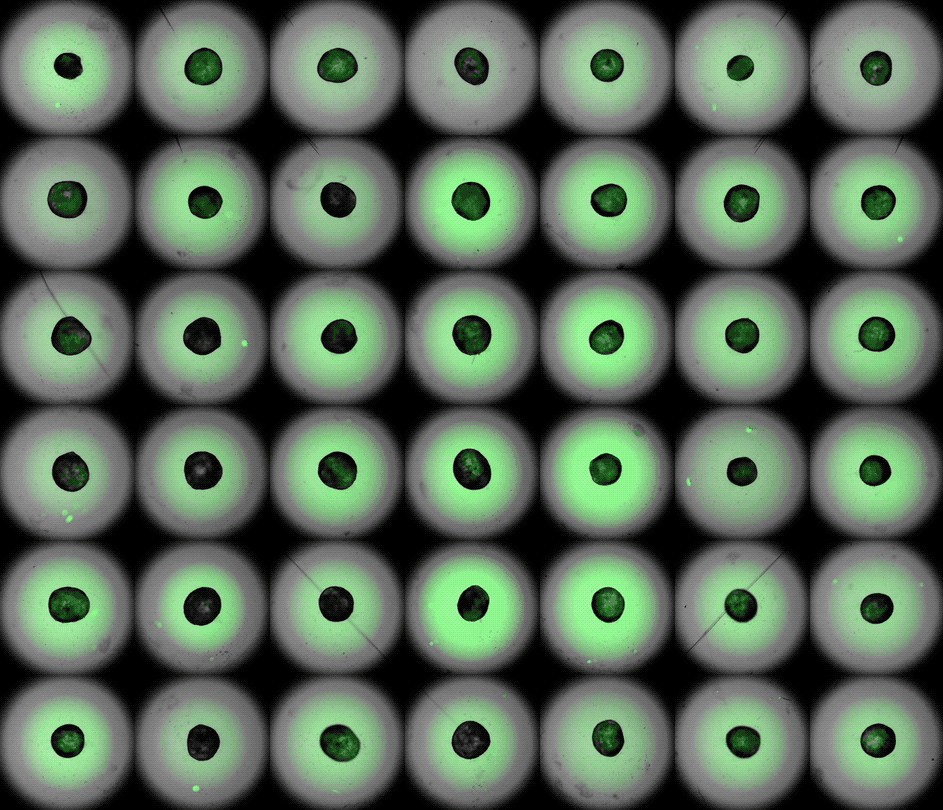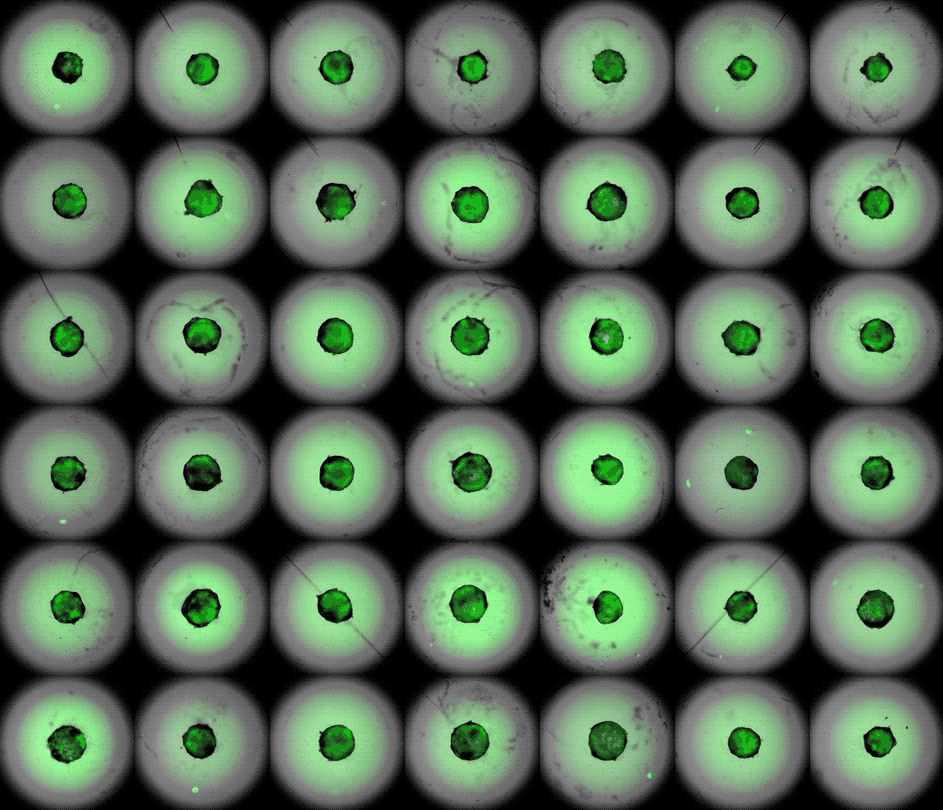
Under the careful eyes of scientists, the stem cells in the laboratory dish were assembled into a small heart “organoid” about the size of a sesame seed and began to “beat” like a real miniature heart.
To guide stem cells to these structures, the research team exposed the cells to a range of proteins and small molecules known to be involved in early humans. heart Development in UterusPublished Thursday (May 20th) in the journal, according to a new study cell.. These proteins and molecules dock with receptors on the cell surface, triggering chain reactions and Stem cells It differentiates into several different cell types found in the heart.
One week after development, the cells were classified into a hollow chamber-like structure that resembled the left ventricle of the left ventricle. heart, The team was found. In addition, the walls of the chamber began to contract rhythmically, mimicking the human heartbeat.
Relation: 11 body parts raised in the lab
“What we are interested in is how the development of the human heart works in essence, and how it fails in the presence of congenital heart disease,” said the Austrian Academy. Sasha Menjan, senior author and group leader at the Institute for Molecular Biotechnology, said. Of science in Vienna.These defects usually occur fairly early pregnancyHowever, scientists cannot directly examine human embryos to see exactly how they develop. “You can’t access this window. It’s essentially a black box,” Menjan told Live Science.
That’s where small organoids come in. These give a glimpse of these early stages of development. The team calls their creation “cardioids” and stands for cardiac organoids.Cardioids may also provide insights into some adults Heart conditionDamaged heart cells regress into a fetal-like state, but cannot regenerate like embryonic cells, Menjan added.
“This study is important in the sense that it started with the embryoid body,” said Ying Mei, an associate professor of bioengineering at Clemson University. , Those who were not involved in the study. In particular, the team succeeded in directing the cells into a hollow chamber structure. This is something that has never been done in the embryoid body.
“As far as I know, this is the first one.”
From cell clumps to cardioid beating
Scientists can also create organoids using an approach called tissue, rather than starting with a mass of stem cells. engineering, This involves building a physical scaffold and introducing cells into that structure. “When you take a tissue engineering approach, you … are building something according to your plans, and you know how the final organs should look,” Menjan said.
“I think both approaches have their own advantages,” May said. For example, Mei and his colleagues created organoids from specific heart cells to simulate a heart attack in a laboratory dish. Nature Biomedical Engineering.. Organoids made from these scaffolds can also be used to screen drugs, such as drugs designed to treat heart damage, before the drug enters clinical trials in animals or humans.
However, while tissue engineering can capture certain aspects of the disease, May says these organoids do not reflect how the actual organs develop in the womb. He said the new cardioid developed by Menjan’s group better captures this developmental process.
To turn a blank stem cell into a small heart, Menjan and his team activated six intracellular molecular pathways. Each pathway represents the spillover effect of intracellular activity that can be triggered by a particular chemical. The team sought to activate these six pathways in different orders and with different amounts of activating chemicals. Eventually, they landed in a combination that gave them small pulsating cardiac organoids.
“In essence, cells had only signals,” meaning activating chemicals, “and they themselves attach. And when they find each other, they have to do what they do. I knew that, “Menjan said. “What we have learned from it is to let the cells do their own thing and minimize interference,” he says, providing only the signals and fuel that cells need to survive in culture.
The cardioid itself resembles a small sphere about 0.04 inches (1 mm) in diameter, which periodically undulates and squeezes the liquid within a hollow center. “This is essentially similar to the 28th day human left ventricle.” pregnancySaid Menjan. He said that the left ventricle, which later pumps oxygenated blood from the heart into the body, is the first structure to develop properly in the heart.
Relation: Birth: Pregnancy stage
With these little hearts in hand, the team conducted experiments to model organoid damage and see if they mimic what happens in a real heart. They used a cold steel rod to freeze part of the cardioid, killing the cells it touched. In response, cardioids sent a fleet of cells called fibroblasts to the injured site, which created a scaffold on the dead cells to keep the organoids intact.
This early stage of the repair process has been observed in animal models, but “this reaction has never been seen. In vitro“Menjan said. “I think I’ve never seen these cardioids because they behave like real organs.”
That said, the team doesn’t know why Cardioid behaves like them, he added. They do not know exactly how or why the six molecular pathways lure stem cells into a heart-like structure. “There are a lot of things we don’t understand yet,” Menjan said. In the future, the team plans to further experiment with these pathways to determine what exact changes will occur in stem cells to form cardioids.
“For me, that’s actually a very interesting question. What makes them form a room?” May said, reflecting his emotions. In addition to explaining these molecular pathways in an easy-to-understand manner, the team is currently working to induce cardioids to develop multiple chambers, such as a real four-chamber heart.
“I don’t see any major barriers to this in practice,” Menjan said. Creating a multi-chamber cardioid allows the team to see the heart valve develop and the septal process in which the heart divides a single chamber into several parts. Menjan said that such cardioids can provide valuable insights into those conditions, as many congenital heart disorders appear around this stage of development.
For now, in current cardioid models, “they mimic the very early stages of heart development,” May said. “many [congenital] The disease begins at a later stage. But you have to start somewhere. ”
Originally published in Live Science.