Health
Single-Dose Monoclonal Antibody Prevents Malaria Infection in Phase II Trial
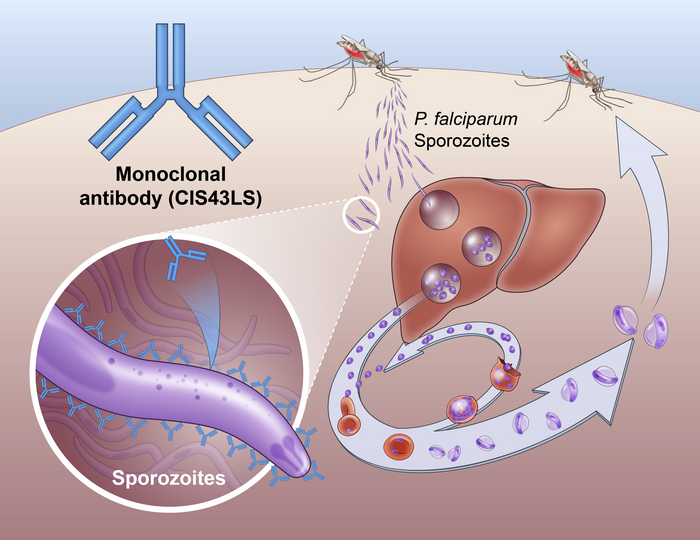
In a phase II clinical trial conducted by the National Institutes of Health, a single dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Mali, Africa. It turns out that The antibody, named CIS43LS, demonstrated protection against infection of up to 88.2% over 24 weeks, demonstrating for the first time that a monoclonal antibody can prevent malaria infection in endemic areas. The results of the trial were recently published. of New England Journal of Medicine (NEJM) and presented at the 2022 American Society for Tropical Medicine and Hygiene Annual Meeting in Seattle.
Anthony S. Fauci, M.D., Ph.D., Director of the National Institute of Allergy and Infectious Diseases (NIAID) said: “These findings suggest that monoclonal antibodies may protect vulnerable groups such as travelers, infants, children and pregnant women from seasonal malaria and help eradicate malaria from defined geographical areas. suggests.”
NIAID sponsored and funded trials led by Peter D. Crompton, MD, MPH and Kassoum Kayentao, MD, MPH, PhD. Crompton is director of the Malaria Infection Biology and Immunity Section of his NIAID Laboratory of Immunogenetics, and Kayentao is a professor at the University of Sciences, Techniques and Technologies (USTTB) (Bamako, Mali).their published report NEJM title is”Safety and efficacy of monoclonal antibodies against malaria in Mali“
Malaria is a mosquito-borne disease that malaria parasite Parasite. According to an estimated 241 million cases of malaria worldwide in 2020. World Health Organization (WHO) An estimated 627,000 people died as a result, mostly children in sub-Saharan Africa. These cases included her more than 11 million pregnant women in Africa, resulting in an estimated 819,000 newborns with low birth weight and increased risk of illness and death.
The only malaria vaccine currently recommended by WHO, RTS,S (Mosquirix), provides partial protection against clinical malaria in children aged 5 to 17 months with 4 doses over 20 months. provides good protection. Other drugs, consisting of small chemical compounds that effectively prevent malaria infection, are also available for infants and travelers. “Malaria control includes early diagnosis and treatment with insecticide-treated nets, artemisinin-based combination therapy, and chemoprevention for high-risk groups such as infants, children exposed to seasonal malaria, and pregnant women. explained the researchers.
However, the need to administer the drug frequently may limit adherence, and the emergence of drug resistance may also limit usefulness. “Chemoprevention is a ‘very important’ tool, but its efficacy may be limited by the challenges of conducting frequent courses of treatment and the emergence of drug resistance,” the researchers said. I’m here. New, rapidly acting, infrequently administered interventions that safely provide strong protection against malaria infection are therefore urgently needed. “Despite these measures, progress in reducing malaria cases and deaths has stalled in recent years and is further threatened by the emergence of insecticide-resistant mosquitoes and drug-resistant parasites … of malaria. New tools are needed to reduce morbidity and mortality and accelerate eradication efforts.”
sending of malaria parasite The parasite that causes malaria is transmitted through the bite of an infected mosquito. Mosquitoes inject parasites into the skin and bloodstream in the form of sporozoites. These migrate to the liver where they mature and multiply. The mature parasites then spread throughout the body through the bloodstream, causing disease. Plasmodium falciparum that is malaria parasite It is the most likely cause of severe malaria infection and can be fatal if not treated promptly.
The reported phase II NIAID-USTTB trial evaluated the safety and efficacy of a single intravenous infusion of CIS43LS monoclonal antibody.This antibody shown before neutralize the sporozoites of Plasmodium falciparum It enters the skin and blood before infecting liver cells. Researchers led by Robert A. Seder, MD isolated a naturally occurring form of this antibody from the blood of his study malaria-vaccinated volunteers and tested the antibody to prolong its residence time in the bloodstream. changed. Seder is Acting Chief Medical Officer and Acting Vice President of the NIAID Vaccine Research Center (VRC), and is also the Director of the VRC’s Cellular Immunity Division.
CIS43LS targets a conserved ‘junction’ epitope on the Plasmodium falciparum circum-protein (PfCSP), a major protein expressed on the surface of sporozoites that are transmitted to humans by mosquitoes, allowing sporozoites to enter hepatocytes. is required for It precedes the red blood cell stage that causes disease. As such, the investigators say, “targeting sporozoites to block infection is an approach to prevent malaria and parasite transmission.”
The reported phase II trial enrolled 369 healthy, nonpregnant adults aged 18 to 55 years in the rural areas of Kalifaboug and Tolod, Mali. Plasmodium falciparum Contagion usually occurs between July and December each year. Investigators noted that the trial had him in two parts. “Part A was an open-label, dose escalation study conducted prior to the malaria season to evaluate the safety and side effect profile of CIS43LS. Part B will evaluate the safety and efficacy of CIS43LS. It was a double-blind, randomized, placebo-controlled trial for
The first part of the study was an open-label, dose escalation study conducted before the start of the malaria season, evaluating the safety and side effect profile of three doses of CIS43LS. 5 milligrams per kilogram of body weight, mg/kg and 40 mg/kg. Antibodies were administered by intravenous infusion to 18 study participants, her 6 participants at each dose level.
A second double-blind portion of the study evaluated the efficacy of two different doses of CIS43LS compared to placebo. A total of 330 participants were randomly assigned to receive antibody doses of either 10 mg/kg, 40 mg/kg, or placebo by intravenous infusion. The research team followed these individuals for her 24 weeks and performed blood tests. Plasmodium falciparum Weekly for the first 28 days, then every 2 weeks. Participants who developed symptomatic malaria during the trial received standard care from the research team.
Also, in both Part A and Part B of the study, artemether-lumefantrine was administered to all participants as a standard, direct-observed course of treatment at enrollment, 7 to 21 days prior to CIS43LS or placebo administration. , eliminated the possibilities. Plasmodium falciparum blood-stage infection, the researchers further noted.
The team followed participants in Part A of the study for 24 weeks. They found that antibody infusion was safe and well-tolerated. “From the administration of CIS43LS to the end of the 24-week study period, no serious adverse events occurred and all unsolicited adverse events were grade 1 or 2 and unrelated to CIS43LS by the investigator. was considered.”
In part B of the study, the team analyzed the efficacy of CIS43LS in two ways.In the primary efficacy analysis based on time to first visit, Plasmodium falciparum In a study of infections during a 24-week trial period, the high dose (40 mg/kg) of CIS43LS was 88.2% effective in preventing infection, and the low dose (10 mg/kg) was 75% effective. It turns out there is. In a secondary efficacy analysis based on Kaplan-Meier estimates of the proportion of participants infected, Plasmodium falciparum During the 24-week trial period, the research team found that the high dose was 76.7% effective in preventing infections and the low dose was 54.2% effective.
Summarizing the results in their paper, the authors report: Plasmodium falciparum Adults were infected during the 6-month malaria season in Mali, during which 78.2% of participants in the placebo group became infected.These data provide proof of concept that monoclonal antibodies with extended half-lives can protect Plasmodium falciparum Infection during a defined period of intense contagion.
“These first field results demonstrate that monoclonal antibodies safely provide high levels of protection against severe malaria infection in healthy adults, and that such interventions are effective against malaria in infants, children and pregnant women. It paves the way for further research to determine whether infections can be prevented,” said Seder. Said. “We hope that monoclonal antibodies will transform malaria prevention in endemic areas.”
The researchers found that a single dose of a monoclonal antibody that prevents infection for up to six months could be viable to complement chemoprevention and other control measures before the malaria season and in early pregnancy in at-risk children. “Overall, our trial provides field data to support the use of monoclonal antibodies as an additional intervention to complement current measures to reduce malaria morbidity and mortality.” To do.”
Seder et al. developed a second antimalarial monoclonal antibody, L9LS, which is much more potent and has a longer half-life than CIS43LS. L9 can be given as a subcutaneous injection (subcutaneous) in smaller doses rather than as an intravenous infusion. Early-stage NIAID trial of L9LS in the United States found Antibodies were safe and prevented malaria infection for 21 days in 15 of 17 healthy adults exposed to Plasmodium falciparum in a carefully controlled environment. His two large NIAID-sponsored phase II trials evaluating the safety and efficacy of her L9LS in infants, children and adults are ongoing. Mali When Kenya.
|
Sources 2/ https://www.genengnews.com/parasitic-diseases/single-dose-of-monoclonal-antibody-prevents-malaria-infection-in-phase-ii-study/ The mention sources can contact us to remove/changing this article |
What Are The Main Benefits Of Comparing Car Insurance Quotes Online
LOS ANGELES, CA / ACCESSWIRE / June 24, 2020, / Compare-autoinsurance.Org has launched a new blog post that presents the main benefits of comparing multiple car insurance quotes. For more info and free online quotes, please visit https://compare-autoinsurance.Org/the-advantages-of-comparing-prices-with-car-insurance-quotes-online/ The modern society has numerous technological advantages. One important advantage is the speed at which information is sent and received. With the help of the internet, the shopping habits of many persons have drastically changed. The car insurance industry hasn't remained untouched by these changes. On the internet, drivers can compare insurance prices and find out which sellers have the best offers. View photos The advantages of comparing online car insurance quotes are the following: Online quotes can be obtained from anywhere and at any time. Unlike physical insurance agencies, websites don't have a specific schedule and they are available at any time. Drivers that have busy working schedules, can compare quotes from anywhere and at any time, even at midnight. Multiple choices. Almost all insurance providers, no matter if they are well-known brands or just local insurers, have an online presence. Online quotes will allow policyholders the chance to discover multiple insurance companies and check their prices. Drivers are no longer required to get quotes from just a few known insurance companies. Also, local and regional insurers can provide lower insurance rates for the same services. Accurate insurance estimates. Online quotes can only be accurate if the customers provide accurate and real info about their car models and driving history. Lying about past driving incidents can make the price estimates to be lower, but when dealing with an insurance company lying to them is useless. Usually, insurance companies will do research about a potential customer before granting him coverage. Online quotes can be sorted easily. Although drivers are recommended to not choose a policy just based on its price, drivers can easily sort quotes by insurance price. Using brokerage websites will allow drivers to get quotes from multiple insurers, thus making the comparison faster and easier. For additional info, money-saving tips, and free car insurance quotes, visit https://compare-autoinsurance.Org/ Compare-autoinsurance.Org is an online provider of life, home, health, and auto insurance quotes. This website is unique because it does not simply stick to one kind of insurance provider, but brings the clients the best deals from many different online insurance carriers. In this way, clients have access to offers from multiple carriers all in one place: this website. On this site, customers have access to quotes for insurance plans from various agencies, such as local or nationwide agencies, brand names insurance companies, etc. "Online quotes can easily help drivers obtain better car insurance deals. All they have to do is to complete an online form with accurate and real info, then compare prices", said Russell Rabichev, Marketing Director of Internet Marketing Company. CONTACT: Company Name: Internet Marketing CompanyPerson for contact Name: Gurgu CPhone Number: (818) 359-3898Email: [email protected]: https://compare-autoinsurance.Org/ SOURCE: Compare-autoinsurance.Org View source version on accesswire.Com:https://www.Accesswire.Com/595055/What-Are-The-Main-Benefits-Of-Comparing-Car-Insurance-Quotes-Online View photos
to request, modification Contact us at Here or [email protected]



